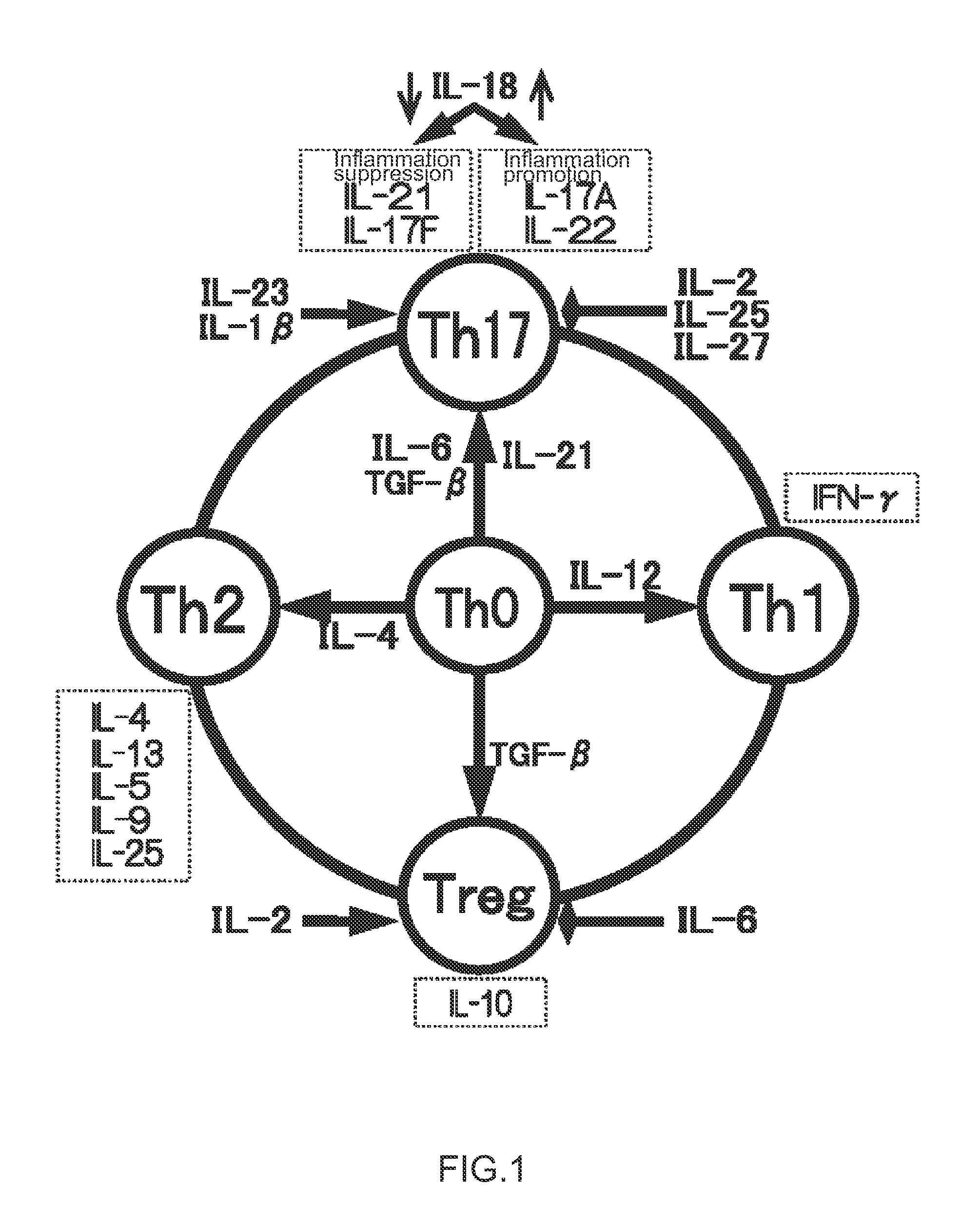
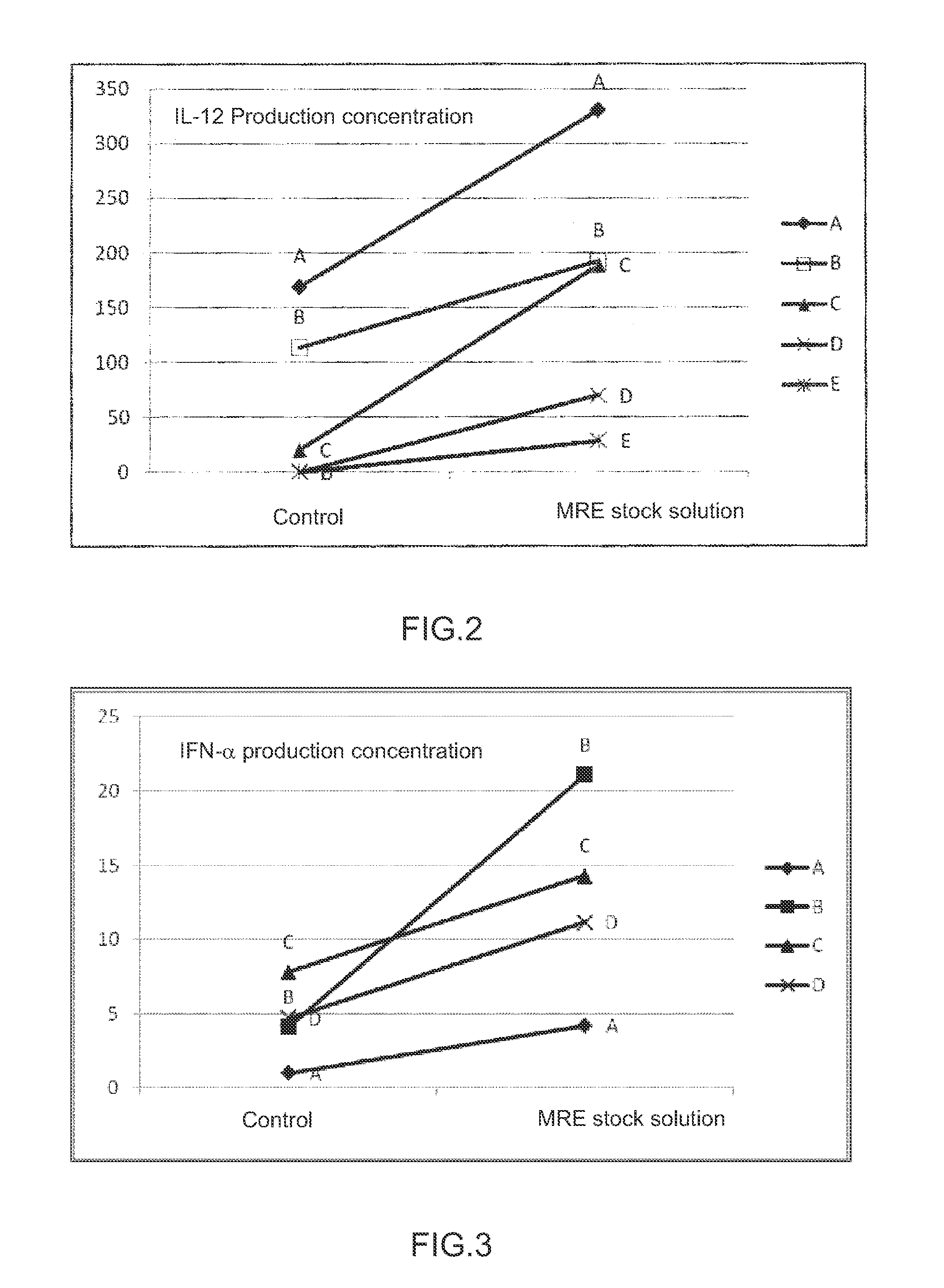
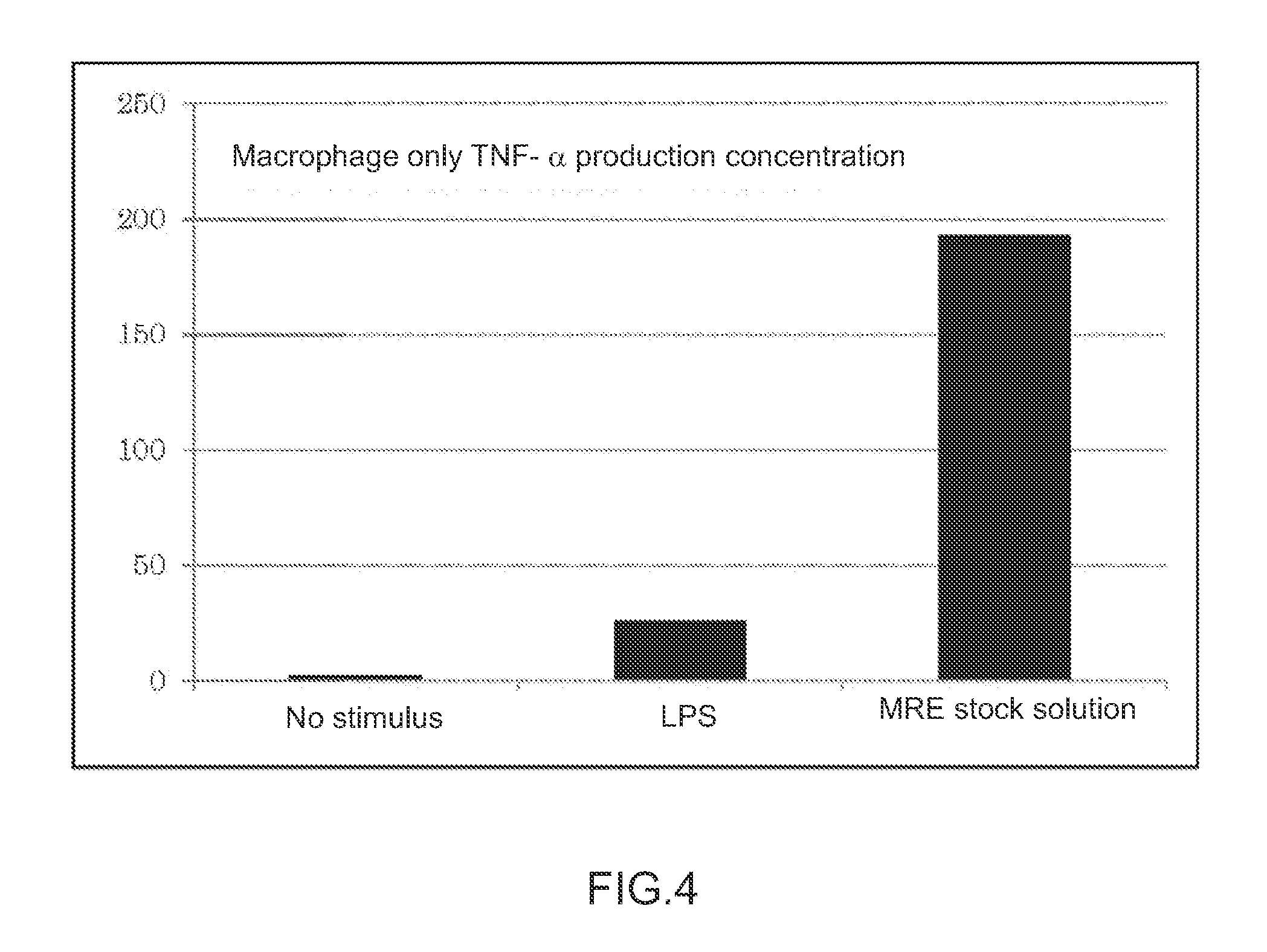
Immunopotentiating composition and process for producing same
a technology of immunopotentiating composition and composition, which is applied in the direction of immunological disorders, antibody medical ingredients, metabolism disorders, etc., can solve the problems of serious problems such as the ineffectiveness of viruses in antibiotics that have been relied upon, and the inability to produce resistant bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of MRE Complex Ligand-Containing Stock Solution by Culturing and Internal Sporulation of an MRE Symbiotic Bacterial Group
[0303]The MRE complex ligand-containing stock solution used in the present invention is prepared by degrading its own bacterial cells of the MRE symbiotic bacteria group down to a low molecular region by blended enzyme groups of the digestive enzyme group secreted from the vegetative cells of the MRE symbiotic bacterial group with a bulk-type enzyme group homologous to the lysosomal enzyme group released along with internal sporulation.
[0304]The culturing of the MRE bacteria group was carried out by a common culturing method known in the art for aerobic gram-positive bacteria. First, a 1.2 m3 culture aeration vessel was filled with 1,000 l water and was aerated. The culture aeration vessel was fed with nutrients: 10 kg fish meal, 10 kg rice bran, 5 kg oil meal, 1 kg broth, along with appropriate amounts of minerals such as magnesium sulfate, silica, an...
trial examples
Clinical Trial Examples
Antiviral Effects
Example 2
AIDS (HIV)
[0307]Twenty persons, selected in an Asian farming village where more than half of its population of 800 are afflicted with HIV due to selling blood, were asked to ingest 200 ml per day of the beverage of the invention for one month. The ratio of CD4 positive T cells to the total T cells (CD4+ / CD3) and the number of CD4 positive T cells per ml of the blood were determined for the three of those who took the beverage and for one who did not but used AIDS drug, by the flow cytometry method, providing results as shown in Table 20.
[0308]A year later all 20 persons who ingested the beverage were well and going about their daily lives including their work. No side effects have been reported at all.
TABLE 20Efficacy of MRE complex ligand-containing stock solution forpatients afflicted with AIDS(Number ofNumber ofCD4+cells) / CD4 positiveTotal numbercells inBeverage ofof T cellsthe bloodthe inventionBefore30 daysBefore30 daysSubjectuse...
example 3
[0309]A male, 48 years of age with hepatitis C virus, who had been treated with interferon and ribavirin, had a viral load of 28,000 / ml, ALT(GPT) of 621, AST(GOT) of 586, which had not dropped significantly, began ingesting the MRE beverage. He took 50 ml per day in addition to Urso. Three months later, the viral load determined with PCR dropped to 12,800 / ml. With the ALT (GPT) dropped to 48 and AST (GOT) to 52, a considerable improvement was made.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com