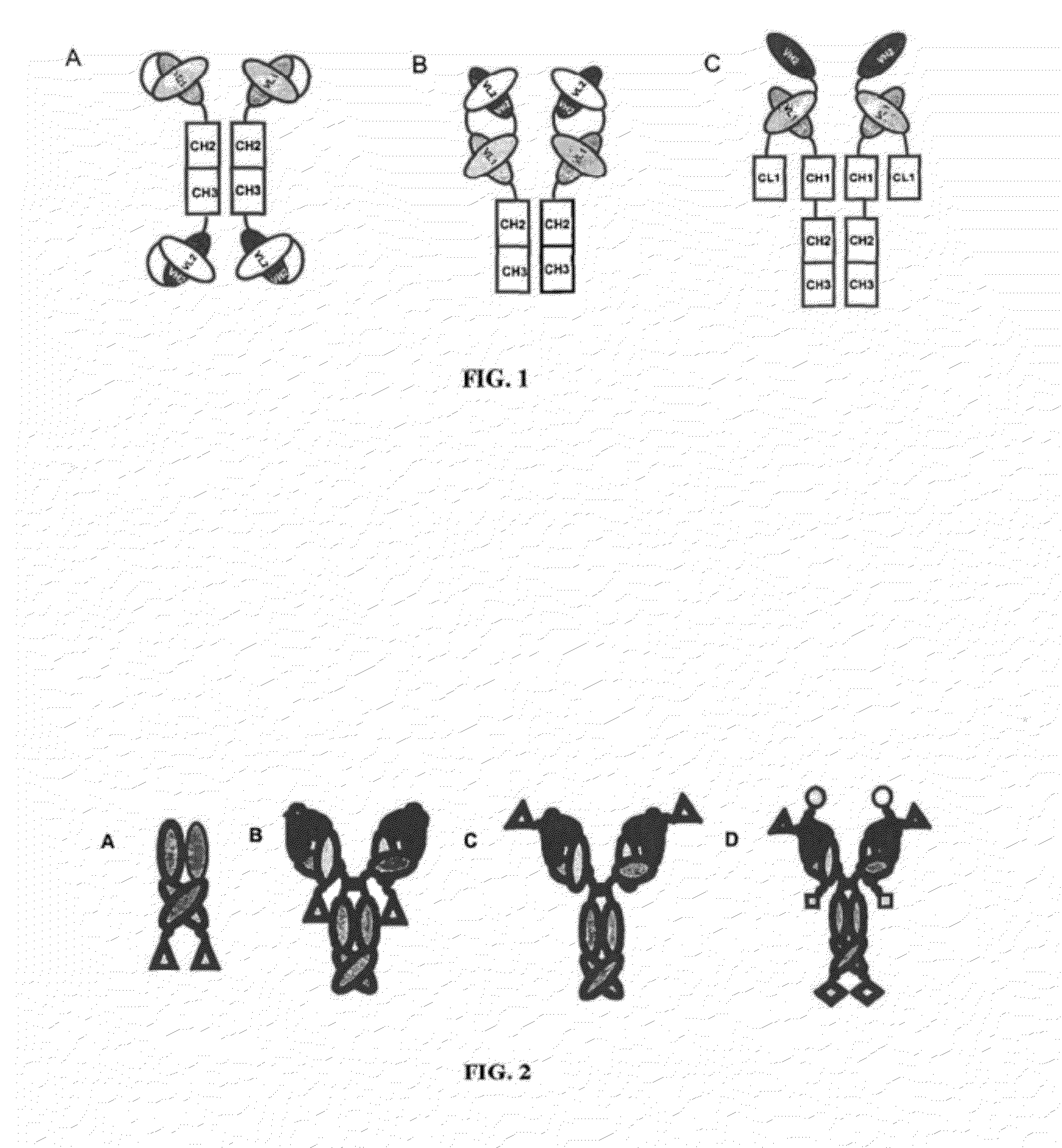Ang-2 Binding Complexes and Uses Thereof
a technology of ang-2 and complexes, applied in the field of ang-2 binding complexes, can solve the problems of difficult recombinant igg-like multi-specific, multi-valent molecules, limited success in the field, and difficulty in rgd-based reporter probes,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Integrin Targeting Antibody-MRD Molecules
[0529]Novel antibody-MRD fusion molecules were prepared by fusion of an integrin αvβ3-targeting peptides to catalytic antibody 38C2. Fusions at the N-termini and C-termini of the light chain and the C-termini of the heavy chain were most effective. Using flow cytometry, the antibody conjugates were shown to bind efficiently to integrin αvβ3-expressing human breast cancer cells. The antibody conjugates also retained the retro-aldol activity of their parental catalytic antibody 38C2, as measured by methodol and doxorubicin prodrug activation. This demonstrates that cell targeting and catalytic antibody capability can be efficiently combined for selective chemotherapy.
example 2
Angiogenic Cytokine Targeting Antibody-MRD Molecules
[0530]Angiogenic cytokine targeting antibody-MRD fusion molecules were constructed. The antibody used was 38C2, which was fused with a MRD containing the 2×Con4 peptide (AQQEECEWDPWTCEHMGSGSATGGSGSTASSGSGSATHQEECEWDPWTCEHMLE (SEQ ID NO:10)). The MRD-containing peptide was fused to either the N- or C-terminus of the light chain and the C-terminus of the heavy chain. Similar results were found with the other Ang2 MRD peptides. Additional Ang2 MRD peptides include: MGAQTNFMPMDNDELLLYEQ FILQQGLEGGSGSTASSGSGSSLGAQTNFMPMDNDELLLY (SEQ ID NO:20) (LM-2x-32); AQQEECEWDPWTCEHMGSGSATGGSGSTASSGSGSATHQEECEWDPWTCEHMLE (SEQ ID NO:10) (2×Con4); AQQEECEFAPWTCEHM (SEQ ID NO:21) ConFA; core XnEFAPWfXn where n is from about 0 to 50 amino acid residues (SEQ ID NO:22); AQQEEC EFAPWTCEHMGSGSATGGSGSTASSGSGSATHQEECEFAPWTCEHMLE (SEQ ID NO:23) (2×ConFA); AQQEECELAPWTCEHM (SEQ ID NO:24) (ConLA); XnELAPWTXn where n is from about 0 to 50 amino acid residues (SEQ...
example 3
Antibody-MRD Fusions with Non-Catalytic Antibodies
[0534]A humanized mouse monoclonal antibody, LM609, directed towards human integrin αvβ3 has been previously described (Rader et. al., PNAS 95:8910-5 (1998)).
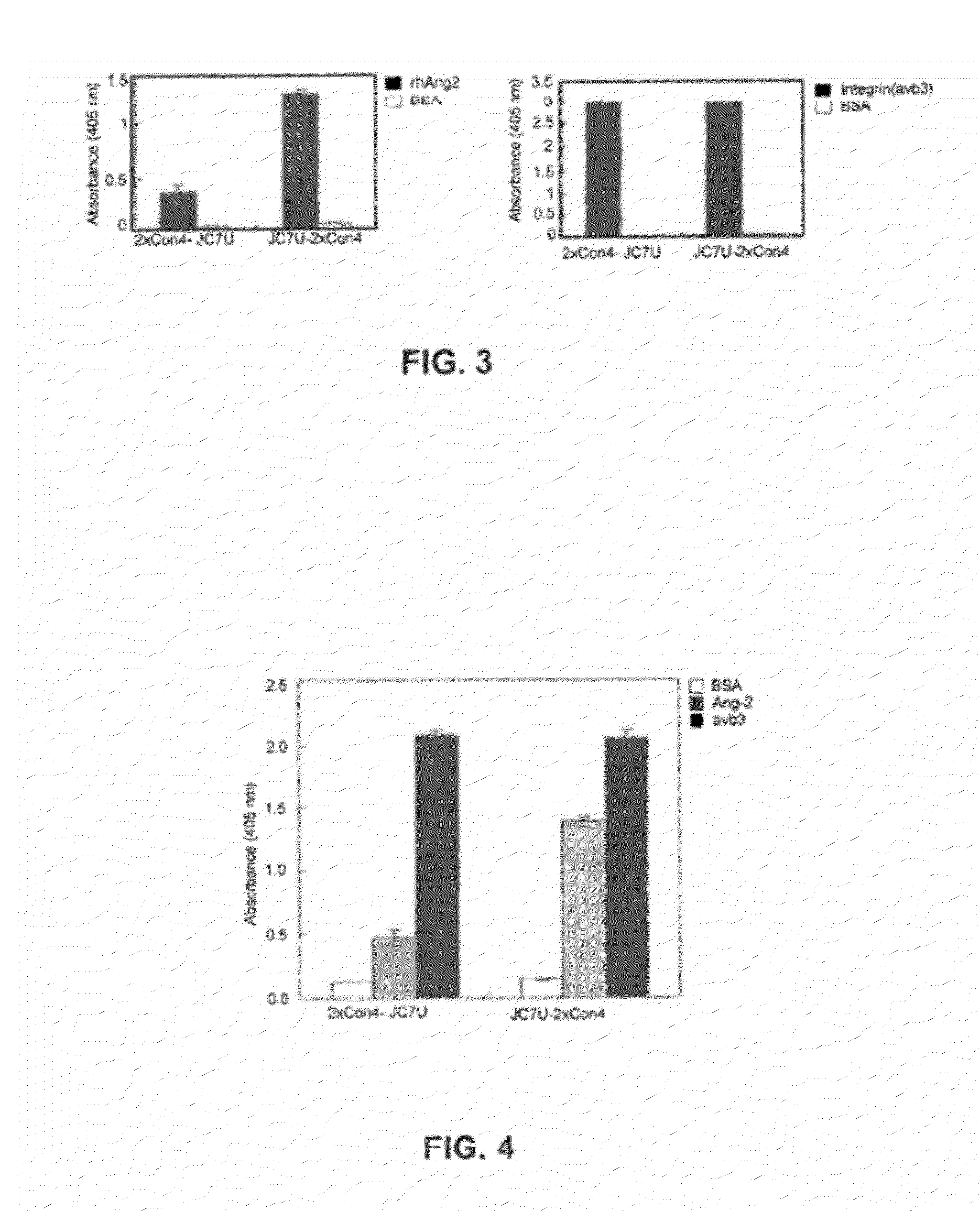
[0535]A human non-catalytic monoclonal Ab, JC7U was fused to an anti-Ang2 MRD containing 2×Con4 (AQQEECEWDPWTCEHMGSGSATGGSGSTASSGSGSATHQEECE WDPWTCEHMLE (SEQ ID NO:10)) at either the N- or C-terminus of the light chain. 2×Con4 (AQQEECEWDPWTCEHMGSGSATGGSGSTASSGSGSATHQEEC EWDPWTCEHMLE (SEQ ID NO:10)) was studied as an N-terminal fusion to the Kappa chain of the antibody (2×Con4-JC7U) and as a C-terminal fusion (JC7U-2×Con4). Both fusions maintained integrin and Ang2 binding. As shown in the left panel of FIG. 3, both antibody constructs (2×Con4-JC7U and JC7U-2×Con4) specifically bound to recombinant Ang2 as demonstrated by ELISA studies. Binding to Ang2, however, is significantly higher with JC7U-2×Con4, which has the 2×Con4 (SEQ ID NO:10) fusion at the C-terminus of the light cha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com