New salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Analytical Methods for the Characterization of the Salts
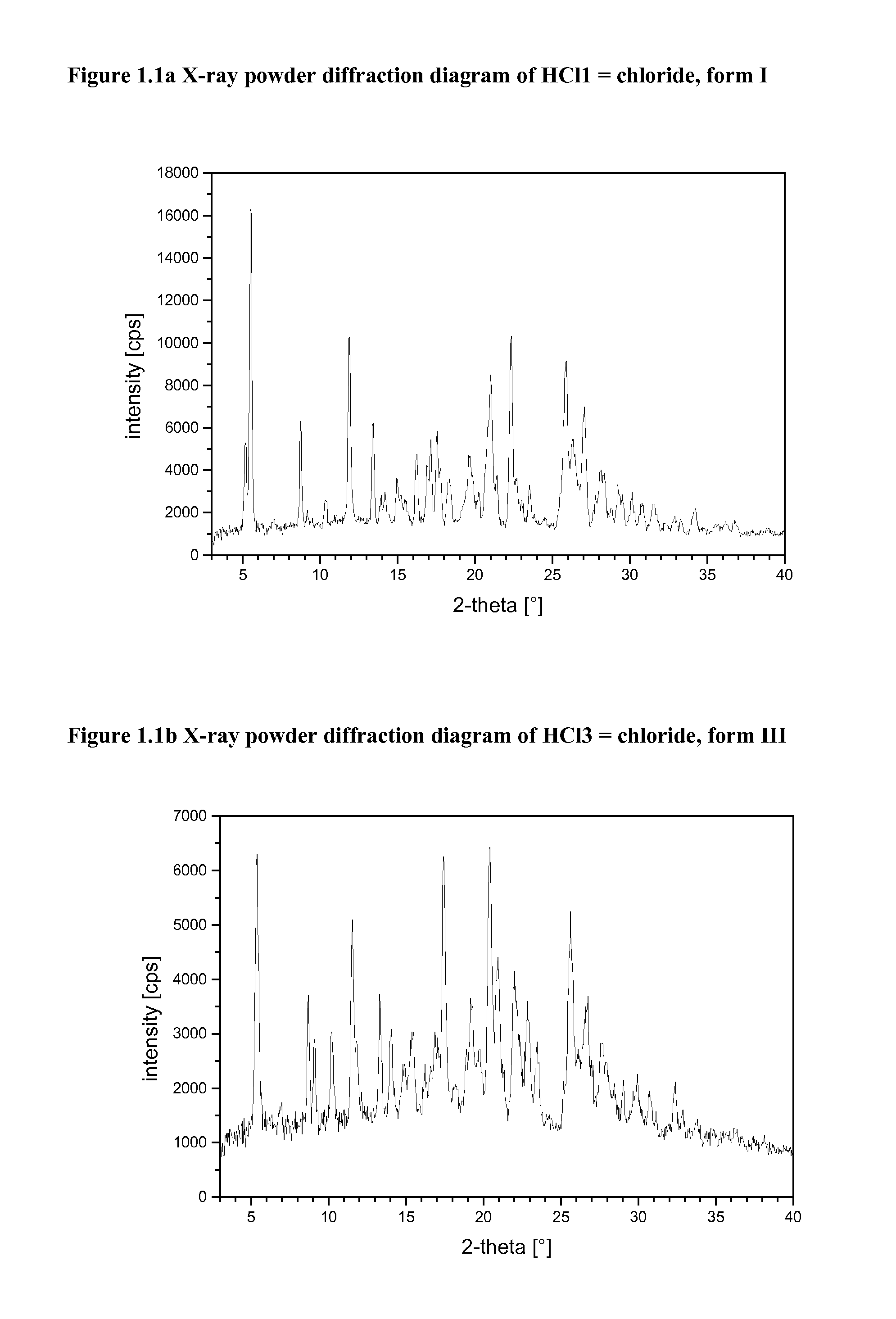
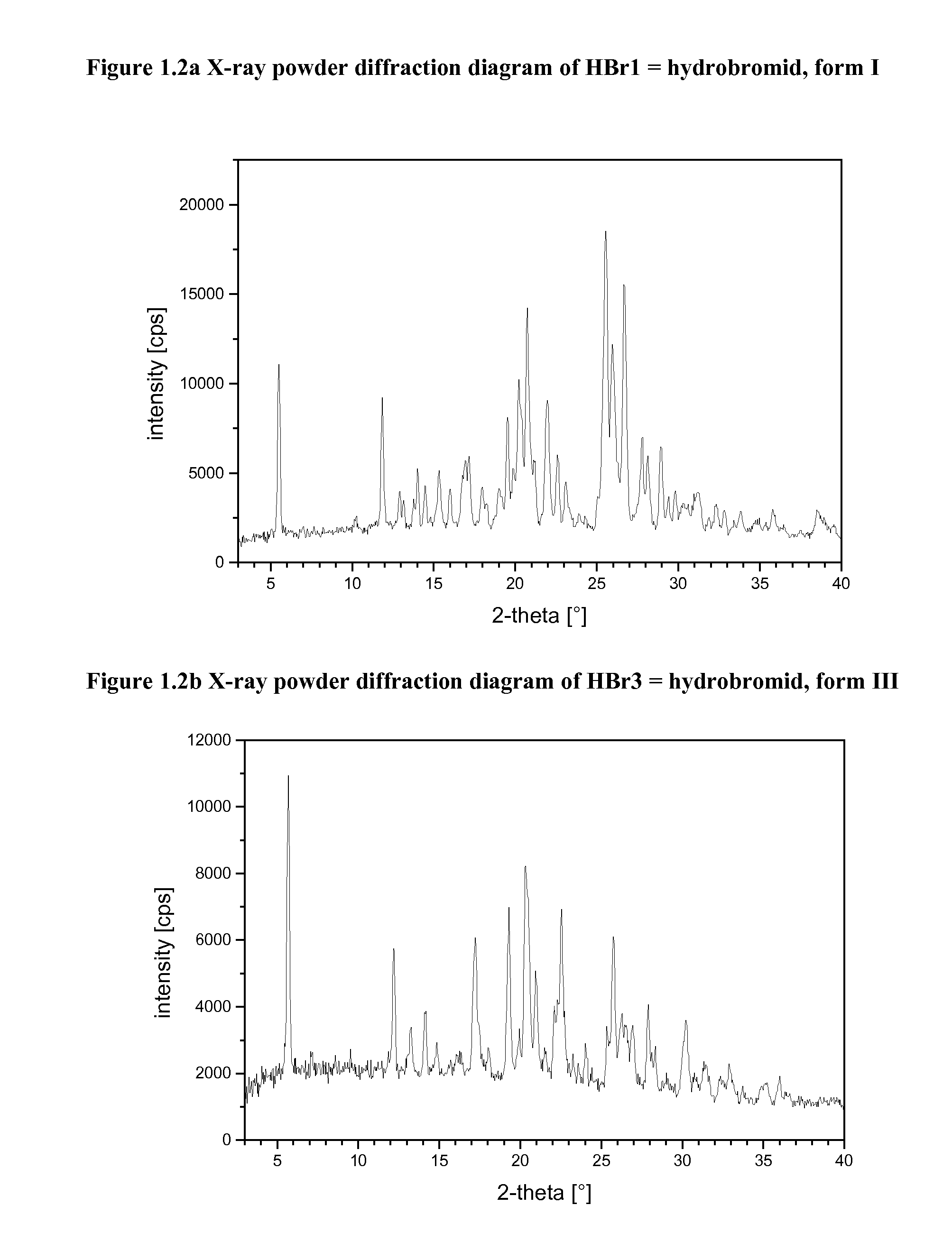
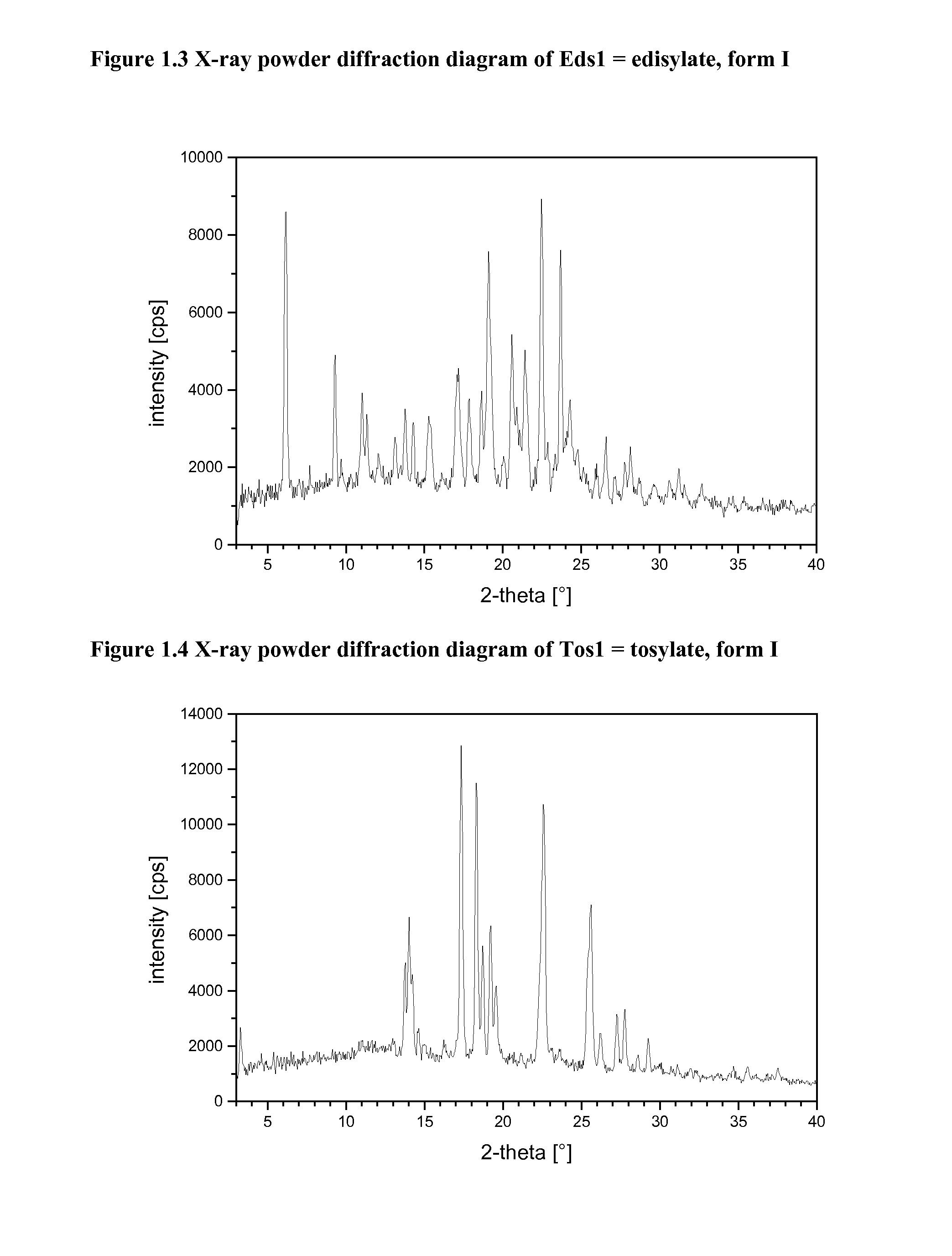
[0104]The harvested crystals may be characterized by X-ray powder diffraction and thermal analysis (DSC). If suitable single crystals grow, single crystal X-ray structure analysis may be performed. The following equipment was used to characterize the crystalline salts forms.
[0105]X-Ray Powder Diffraction (=XRPD)
[0106]XRPD patterns were obtained using a high throughput XRPD set-up. The plates were mounted on a Bruker GADDS diffractometer equipped with a Hi-Star area detector. The diffractometer was calibrated using Silver Behenate for the long d-spacings and corundum for the short d-spacings.
[0107]The data collection was carried out at room temperature using monochromatic CuKα radiation in the region of 2Θ between 1.5 and 41.5°. The diffraction pattern of each well was collected with an exposure time of 3-4 minutes.
[0108]Single Crystal X-Ray Structure Analysis
[0109]Suitable single crystals were selected and glued to a glass fibr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com