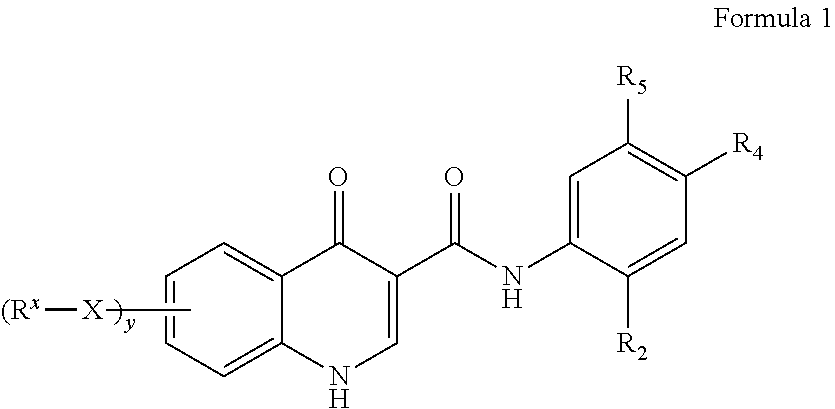
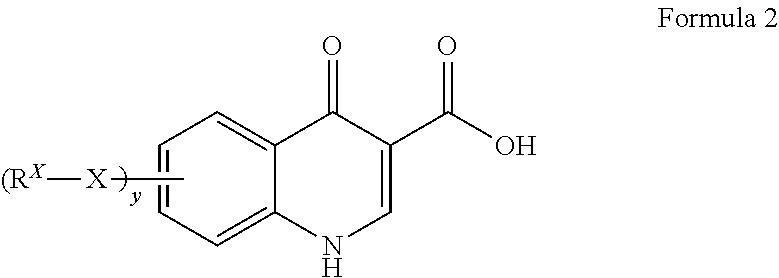
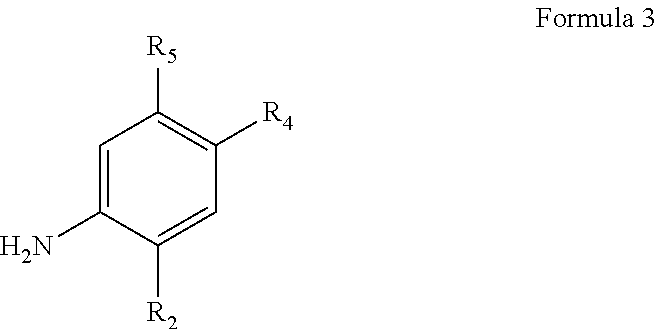
Process for making modulators of cystic fibrosis transmembrane conductance regulator
a technology of transmembrane conductance and modulator, which is applied in the field of making modulators of cystic fibrosis transmembrane conductance regulator, can solve the problems of imbalance in ion and fluid transport, no cure, and individuals with two copies of the cf associated gene suffer from the debilitating and fatal effects of cf, so as to reduce and the effect of reducing the volume of the organic phas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Total synthesis of N-(2-tert-butyl-5-hydroxy-4-(1-hydroxy-2-methylpropan-2-yl)phenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide (27)
[0314]The overall scheme of the synthesis of compound 27 is shown below, followed by the procedure for the synthesis of each synthetic intermediate.
Procedure for the Preparation of 2-hydroxy-5-tert-butylbenzaldehyde (2)
[0315]
[0316]To a stirred solution of compound 1 (700 g, 4.66 mol) in CH3CN (7.0 L) was added MgCl2 (887 g, 9.32 mol), Para-Formaldehyde (1190 g) and TEA (2.5 L, 17.9 mol) under N2. The mixture was heated to reflux for 5 hours. After cooling to room temperature, 2 L ice water was added to the mixture, followed by 6 L of 3 M HCl (aq). The suspension was left stirring until the solution became clear. The organic layer was separated and the aqueous layer was extracted with MTBE (3 L×3). The organic layers were combined and concentrated to dryness. The residue was dissolved in MTBE (4000 mL), washed with water (1000 mL×2) and brine (1000 mL), d...
example 2
Alternative Total Synthesis of N-(2-tert-butyl-5-hydroxy-4-(1-hydroxy-2-methylpropan-2-yl)phenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide (27)
[0343]
Procedure for the Preparation of methyl 2-(5-tert-butyl-2-hydroxy-4-nitrophenyl)-2-methylpropanoate (38)
[0344]
[0345]A mixture of 2-bromo-4-tert-butyl-5-nitrophenol (15.00 g, 54.72 mmol), bis(tri-tert-butylphospine)palladium(0) (1.422 g, 2.783 mmol), zinc fluoride (2.82 g, 27.27 mmol), methyl trimethylsilyl dimethylketene acetal (MTDA) (19.35 g, 111.0 mmol), and dimethylformamide (150 mL) was heated at 70° C. for 18 h. The mixture was cooled to room temperature and diluted with water. After stirring for one hour, the aqueous phase was extracted with MTBE. The organic layer was dried in vacuo to afford the crude product as a brown solid. Purification of the product was accomplished by trituration in n-heptane. 1H-NMR (400 MHZ, DMSO-d6) δ 10.38 (s, 1H); 7.37 (s, 1H); 6.79 (s, 1H); 3.54 (s, 3H); 1.45 (s, 6H); 1.32 (s, 9H)
Procedure for the P...
example 3
Total Synthesis of 2-(5-tert-butyl-2-hydroxy-4-(4-oxo-1,4-dihydroquinoline-3-carboxamido)phenyl)-2-methylpropanoic acid (28)
[0354]
Procedure for the Preparation of 2-(5-tert-butyl-2-hydroxyphenyl)-2-methylpropanenitrile (15)
[0355]
[0356]Pd (OH)2 / C (2.0 g) and compound 7 (20.0 g, 0.104 mol) were stirred in MeOH (150 mL) at room temperature under hydrogen at 10 psi pressure for 16-18 hours. The mixture was then filtered through a pad of Celite®, and the filtrate was concentrated to give compound 15, which was used in the next reaction without further purification. 1H NMR (DMSO-d6; 400 MHz) δ 9.83 (s), δ 7.24 (s), δ 7.18 (m), δ 6.80 (m), δ 1.71 (s), δ 1.24 (s).
Procedure for the Preparation of 4-tert-butyl-2-(2-cyanopropan-2-yl)phenyl methyl carbonate (16)
[0357]
[0358]To a stirred mixture of compound 15 (126.6 g, 0.564 mol), DMAP (6.0 g) and DIEA (188 g, 1.46 mol) in anhydrous DCM (1500 mL) was added dropwise methyl chloroformate (110 g, 1.17 mol) in anhydrous DCM (300 mL) at 0° C. within ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| reaction temperature | aaaaa | aaaaa |
| reaction temperature | aaaaa | aaaaa |
| reaction temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com