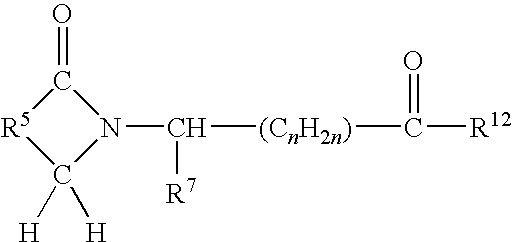
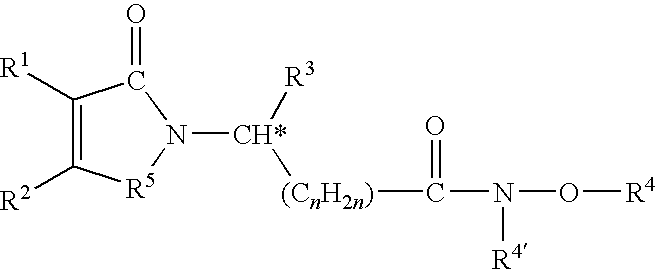
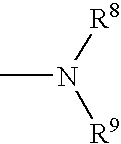METHODS FOR TREATMENT OF MULTIPLE MYELOMA USING CYCLOPROPANE CARBOXYLIC ACID {2-(Is)-(3-eTHOXY-4METHOXY-PHENYL)-2-METHANESULFONYL-ETHYL}-3-OXO-2.3-DIHYDRO-1H-ISOINDOL-4-YL}-AMIDE
a technology of cyclopropane carboxylic acid and multiple myeloma, which is applied in the direction of antimycotics, peptide/protein ingredients, depsipeptides, etc., can solve the problems of only effective radiation therapy, surgery may not completely remove neoplastic tissue, and patients' applicability poses significant drawbacks, so as to prolong the time of remission and prevent or prolong the recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] A first embodiment of the invention encompasses methods of treating, managing, or preventing cancer which comprises administering to a patient in need of such treatment or prevention a therapeutically or prophylactically effective amount of a selective cytokine inhibitory drug of the invention, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof.
[0024] In particular methods encompassed by this embodiment, the selective cytokine inhibitory drug is administered in combination with another drug (“second active agent”) or method of treating, managing, or preventing cancer. Second active agents include small molecules and large molecules (e.g., proteins and antibodies), examples of which are provided herein, as well as stem cells. Methods, or therapies, that can be used in combination with the administration of the selective cytokine inhibitory drug include, but are not limited to, surgery, blood transfusions, immunotherapy, biolog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com