Methods and pharmaceutical compositions useful for treating psoriasis
a technology of psoriasis and composition, applied in the direction of biocide, plant growth regulator, plant ingredients, etc., can solve the problems of limited use of psoriasis treatment agents and practical problems of applying such topical formulations to patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
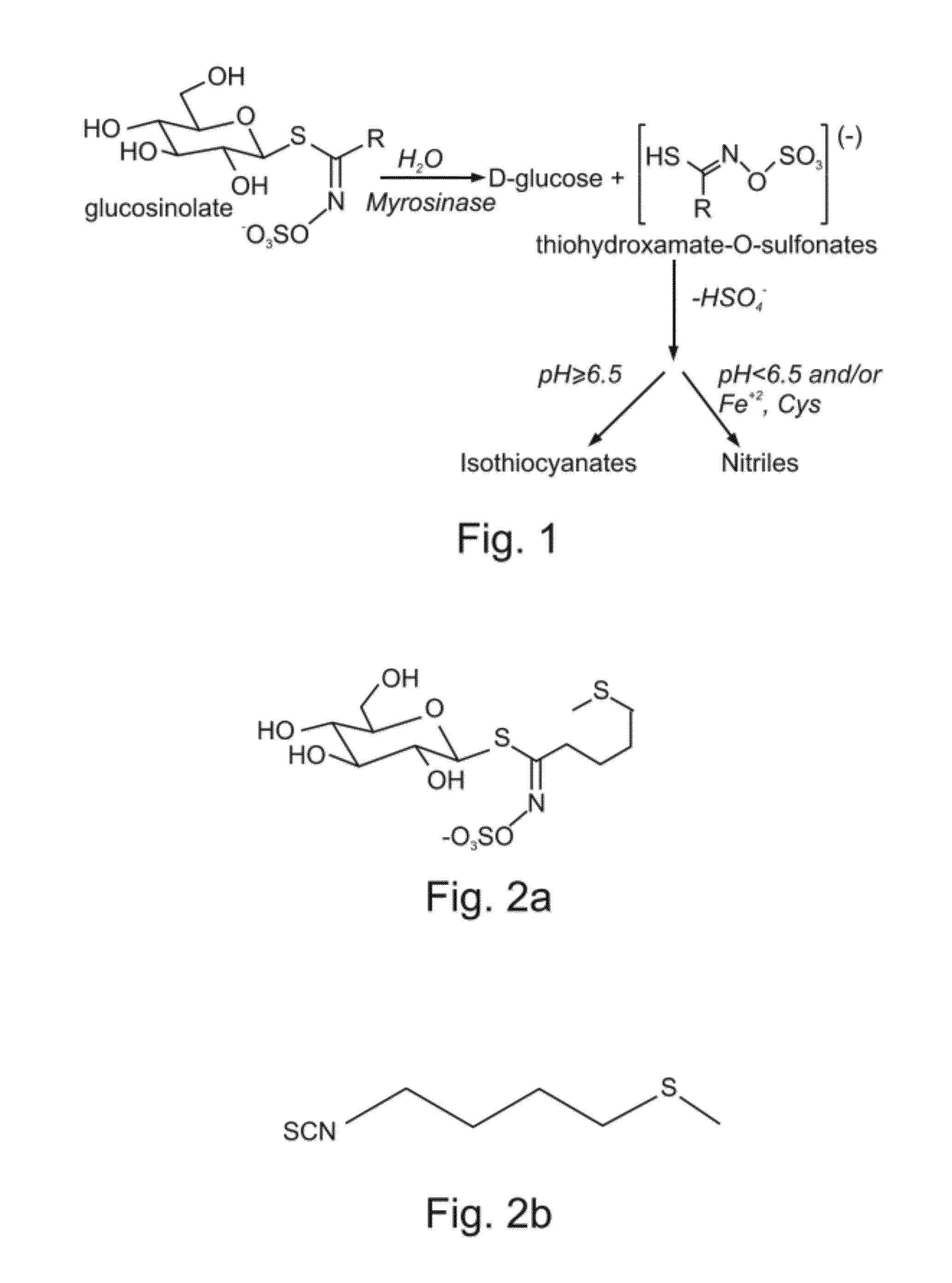
Plant Seed Extract Containing 4-(Methylthio) Butylisthiocyanate is Capable of Treating Psoriasis Lesions
[0191]A novel composition useful for treating psoriasis lesions was identified by the present inventor, as follows.
[0192]Materials and Experimental Methods
[0193]Eruca Sativa plant extract—An alcoholic extract made from the ripe seeds of rocket (Eruca Sativa Miller) plant (at a 1:1 ratio) was purchased from The Herbal Apothecary (Syston Leicester, England).
[0194]Preparation of an Eruca Sativa plant extract cream—The alcoholic plant extract of E. Sativa was mixed at a 15% final concentration with a base cream to form an emulsion (Base cream Batch No. 50402, C.T.R. Tel Aviv, Israel). The cream was water base to avoid the use of fatty acids which tend to relieve the dryness characterizing the psoriasis lesions.
[0195]Study subjects and application of cream—Psoriasis patients suffering from various degrees of psoriasis lesions participated in the study. Application of the Eruca Sativa p...
example 2
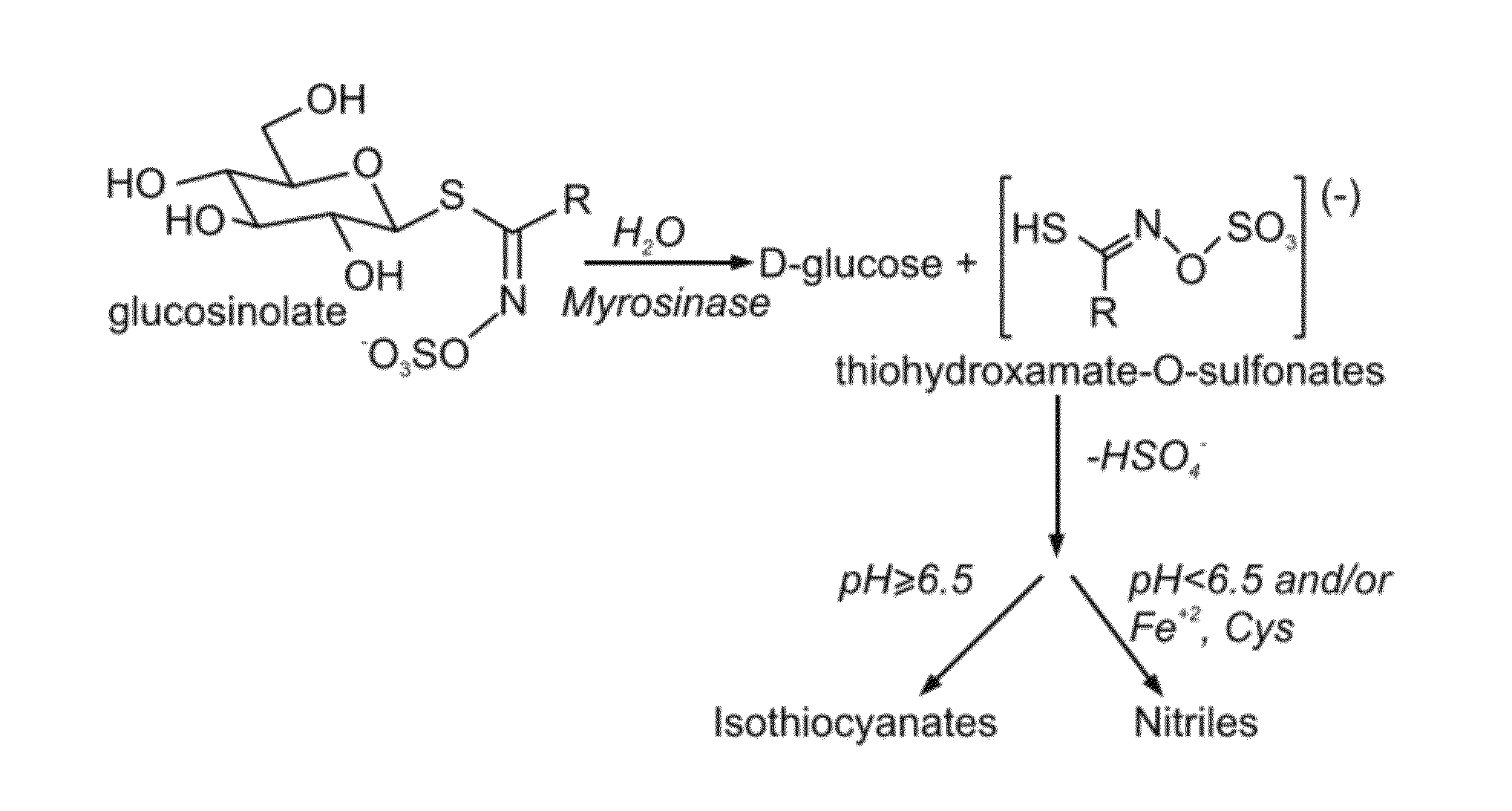
Enzymatic Preparation of 4-Methyl-Thio-Butyl-Isothiocyanate from Glucoerucin Enriched Extract of Eruca Sativa
[0204]To efficiently treat psoriasis, the present inventor further isolated the 4-methyl-thio-butyl-isothiocyanate from an enriched fraction of glucoerucin extract prepared from the ripe seeds of rocket (Eruca sativa), as follows.
[0205]Materials and Experimental Methods
[0206]Extraction of glucoerucin from the ripe seeds of rocket (Eruca sativa)—To extract glucoerucin from the ripe seeds of rocket (Eruca sativa), 1 gram of seeds was heated for 24 hours at 100° C., homogenized for 5 minutes in 70% ethanol, following which the homogenate was heated for 30 minutes at 70° C., cooled down to room temperature and centrifuged for 15 minutes at 17000 g. The ethanol from the obtained supernatant was evaporated and after partial evaporation the remaining water fraction was filtered using 0.2 / 0.45 filter. The presence of glucoerucin was identified using HPLC(HP 1100) connected to UV / VIS...
example 3
Cytotoxicity Effects of 4-Methyl-Thio-Butyl-Isothiocyanate (MTBITC) on Hyperproliferative Cells
[0213]The present inventor further determined the effect of the glucoerucin hydrolysis products which contain 4-methyl-thio-butyl-isothiocyanate on the proliferation of normal or hyperproliferative cells, as follow.
[0214]Materials and Experimental Methods—
[0215]Cell lines—The human keratinocyte cell line [HaCaT; (Boukamp et al 1988 The Journal of cell biology 106:761-771] are considered immortal, reveal a heteroploid stemline with specific stable marker chromosomes, but are not tumorigenic. They have a remarkable capacity for normal differentiation and thus offer a suitable and stable model for studying keratinocyte cells. The Jurkat T-leukemia cells are leukemia cells known to be affected in their cell-cycle progression and apoptosis induction by 4-(methylthio)butylisothiocyanate (Fimognari et al., 2004 Investigational New Drugs 22:119-129).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com