Therapeutic agent for pulmonary fibrosis
a technology for pulmonary fibrosis and therapeutic agents, which is applied in the direction of biocide, organic chemistry, drug compositions, etc., can solve the problems that none of these agents are satisfactory, and the progression of idiopathic interstitial pneumonia into pulmonary fibrosis, so as to prevent the onset of pulmonary fibrosis, and reduce the risk of pulmonary fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of siRNA
[0077]Three types of siRNA targeted at gp46 (GenBank Accession No. M69246), which is a rat homologue of human HSP47, and a random siRNA control were purchased from Hokkaido System Science Co., Ltd. Each siRNA consists of 27 bases overhanging on the 3′ side, and the sequences are as follows.
Sequence A:(sense, SEQ ID NO: 1)5′-GUUCCACCAUAAGAUGGUAGACAACAG-3′(antisense, SEQ ID NO: 2)5′-GUUGUCUACCAUCUUAUGGUGGAACAU-3′Sequence B:(sense, SEQ ID NO: 3)5′-CCACAAGUUUUAUAUCCAAUCUAGCAG-3′(antisense, SEQ ID NO: 4)5′-GCUAGAUUGGAUAUAAAACUUGUGGAU-3′Sequence C:(sense, SEQ ID NO: 5)5′-CUAGAGCCAUUACAUUACAUUGACAAG-3′(antisense, SEQ ID NO: 6)5′-UGUCAAUGUAAUGUAAUGGCUCUAGAU-3′Random siRNA:(sense, SEQ ID NO: 7)5′-CGAUUCGCUAGACCGGCUUCAUUGCAG-3′(antisense, SEQ ID NO: 8)5′-GCAAUGAAGCCGGUCUAGCGAAUCGAU-3′
[0078]Furthermore, siRNA that was labeled on the 5′ side with the fluorescent dye 6′-carboxyfluorescein (6-FAM) was also prepared.
example 2
Preparation of siRNA-Containing VA-Bound Liposome
[0079]As a liposome, a cationic liposome containing DC-6-14, cholesterol, and DOPE at a molar ratio of 4:3:3 (Lipotrust, Hokkaido System Science Co., Ltd.) was used. 10 nmol of liposome and 20 nmol of vitamin A (VA: all-trans retinol, Sigma) were mixed in DMSO using a 1.5-mL tube, then dissolved in chloroform, evaporated once, and then suspended in PBS (phosphate buffered saline). Subsequently, the siRNA (10 μg / mL) obtained in Example 1 and the liposome suspension were mixed at a ratio of 1:1 (w / w). Free VA and siRNA contained in the liposome suspension thus obtained were removed by a micropartition system (Sartorion VIVASPIN 5000MWCO PES), thus giving an siRNA-containing VA-bound liposome (VA-lip-siRNA). The amount of VA added and the amount of VA contained in the purified liposome were measured by HPLC, and the proportion of VA bound to the liposome was examined; as a result, it was found that the majority of the VA (95.6±0.42%) was...
example 3
In Vivo Anti-Pulmonary-Fibrosis Activity of siRNA-Containing Va-Bound Liposome
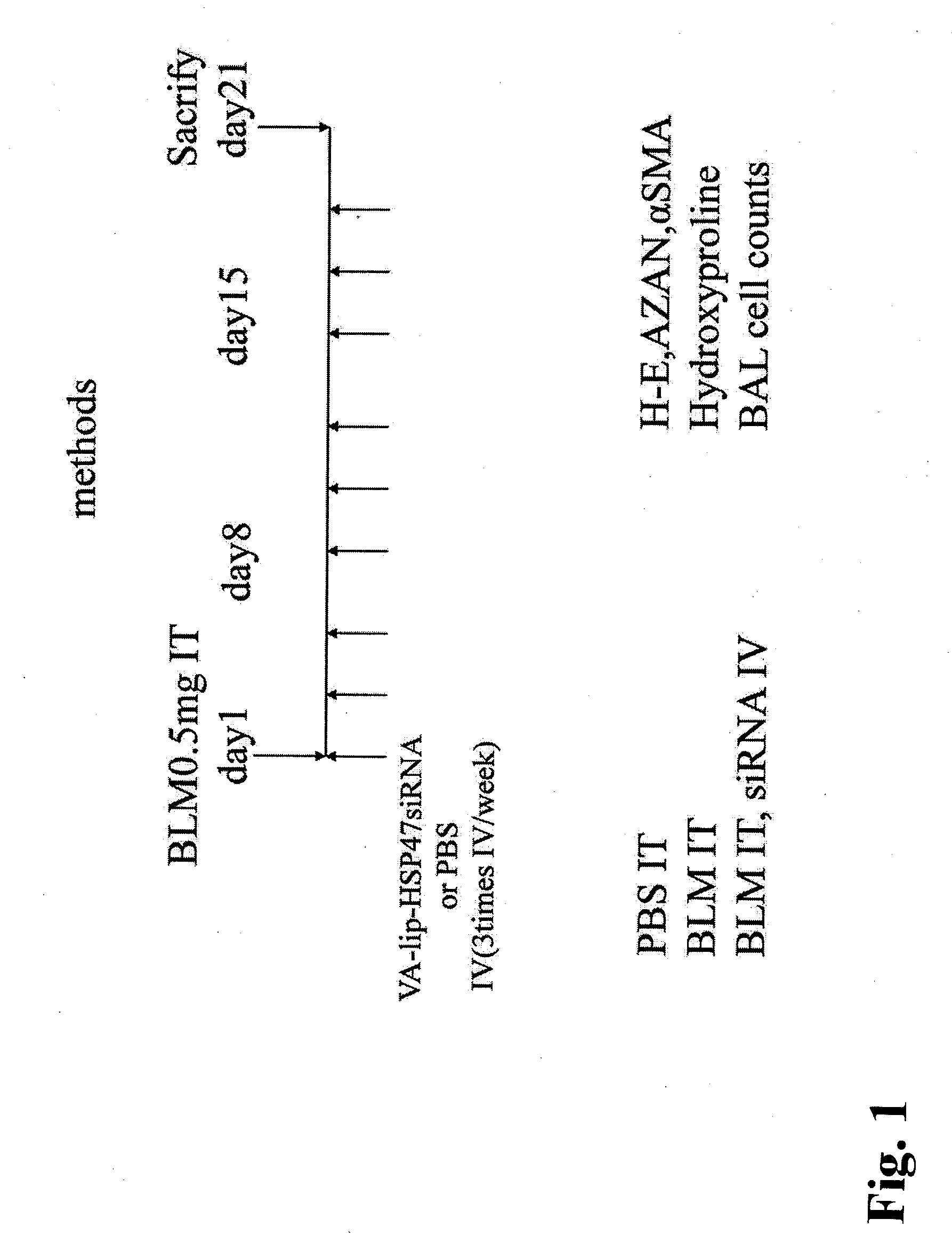
[0080](1) Induction of pulmonary fibrosis and administration of drug
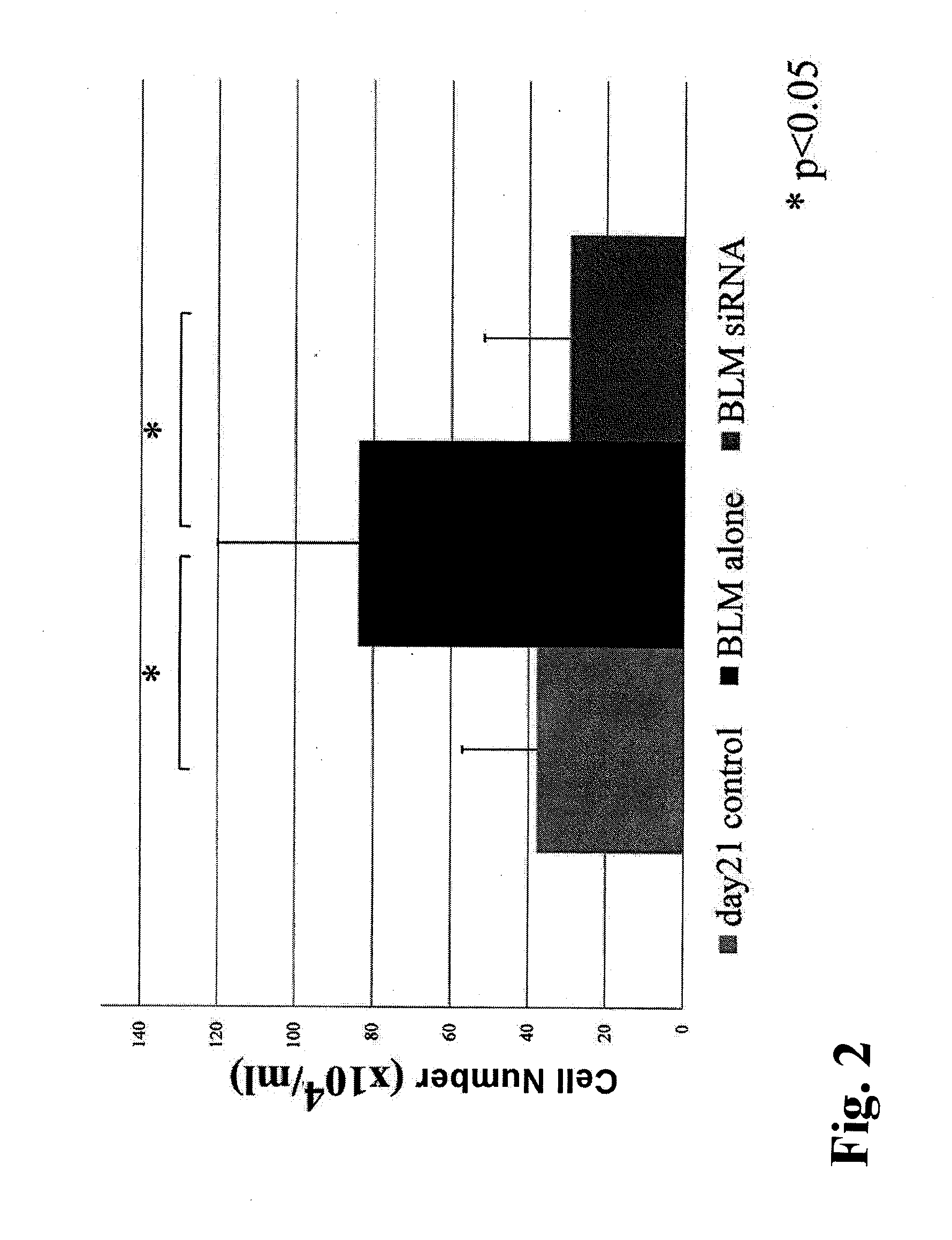
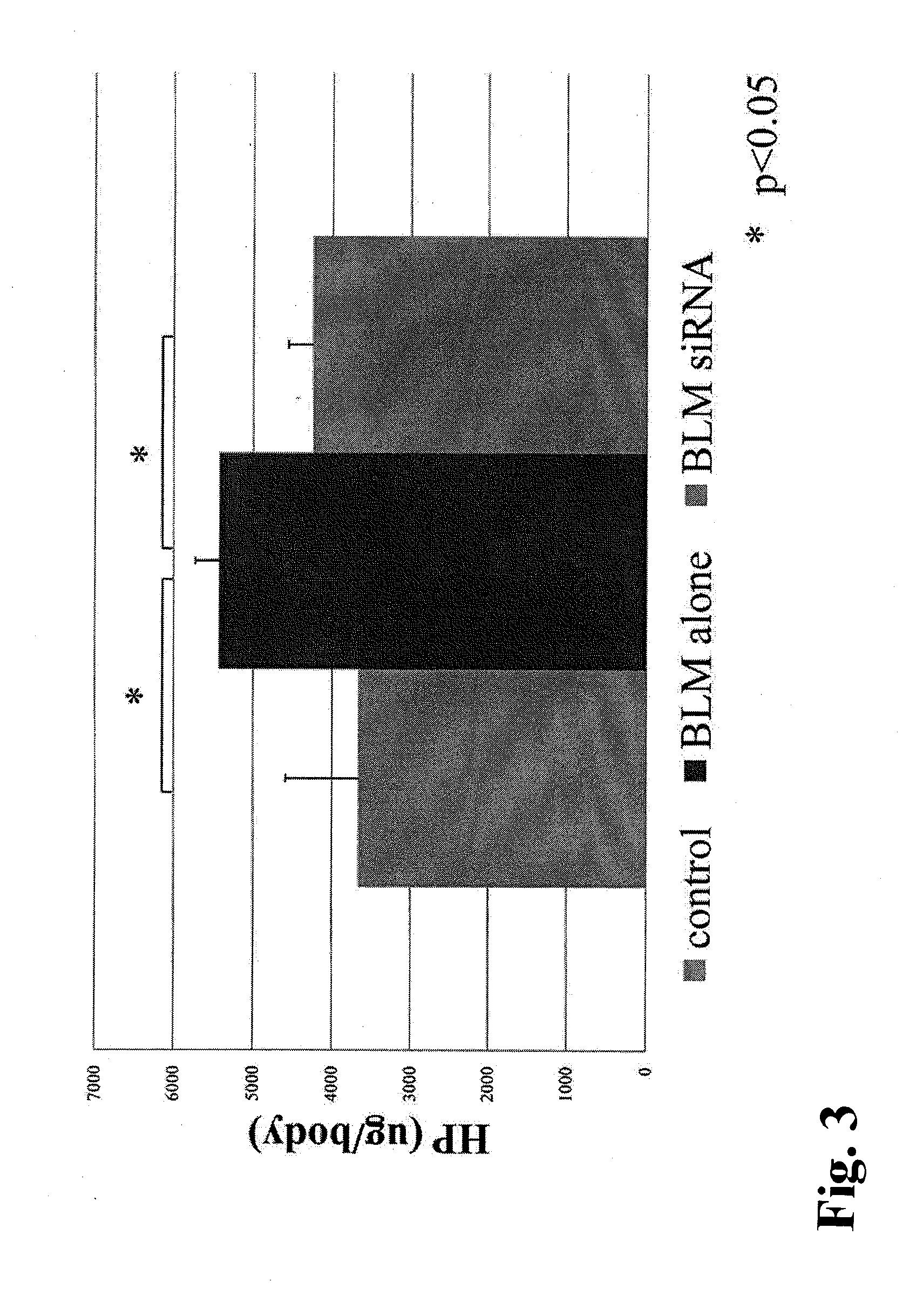
[0081]Male S-D rats (6 rats / group, 4 weeks old, Charles River Laboratories Japan, Inc.) were administered once with 0.5 mg bleomycin (BLM) dissolved in 0.5 cc of physiological saline into the lung intratracheally by intratracheal cannulation under anesthesia, to produce a bleomycin pulmonary fibrosis model. With this method, a significant fibrosis occurs in the lung generally after approximately 3 weeks. The VA-lip-siRNA prepared in Example 2 (0.75 mg / kg as an amount of siRNA, 1 ml / kg in volume, i.e., 200 μl for a rat of 200 g) or PBS (1 ml / kg in volume) was administered to the rats via the tail vein, starting from the day of administration of bleomycin, at a frequency of 3 times / week. The rats were sacrificed 21 days after the bleomycin administration, and bronchoalveolar lavage (BAL) fluid was analyzed, hydroxyproline in the lung was qua...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com