Isolated populations of adult renal cells and methods of isolating and using same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Adherent hFK Cell Cultures
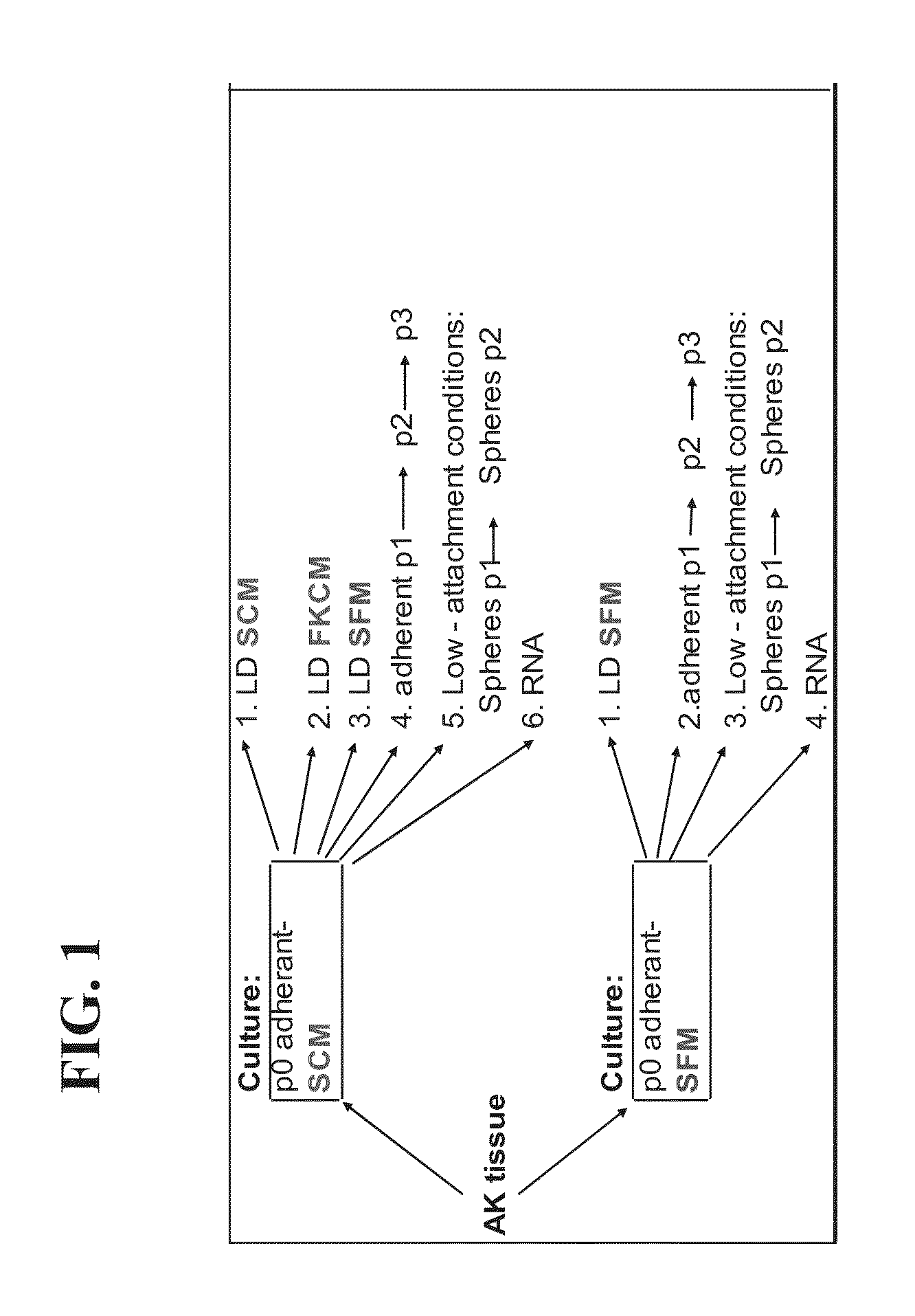
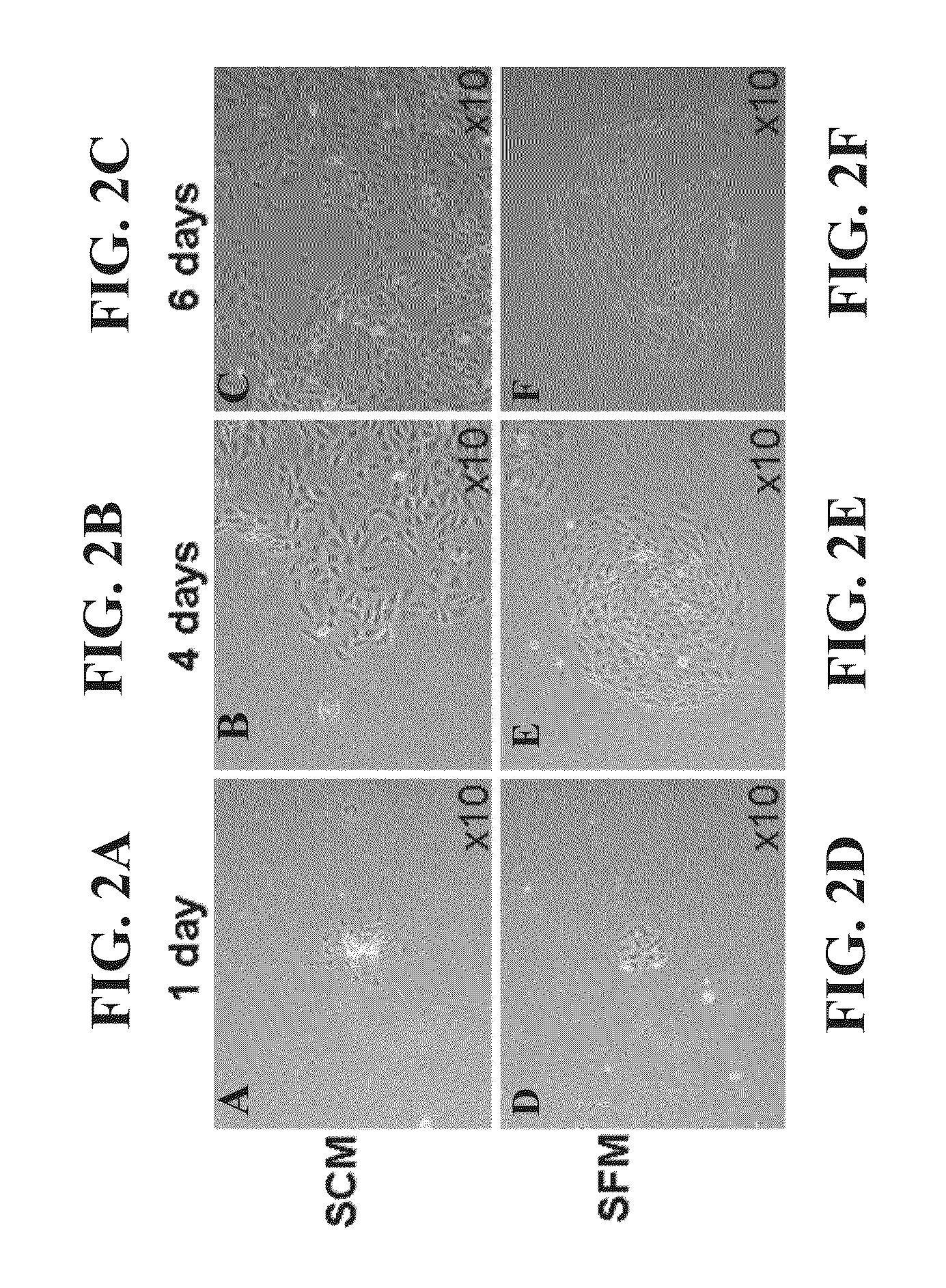
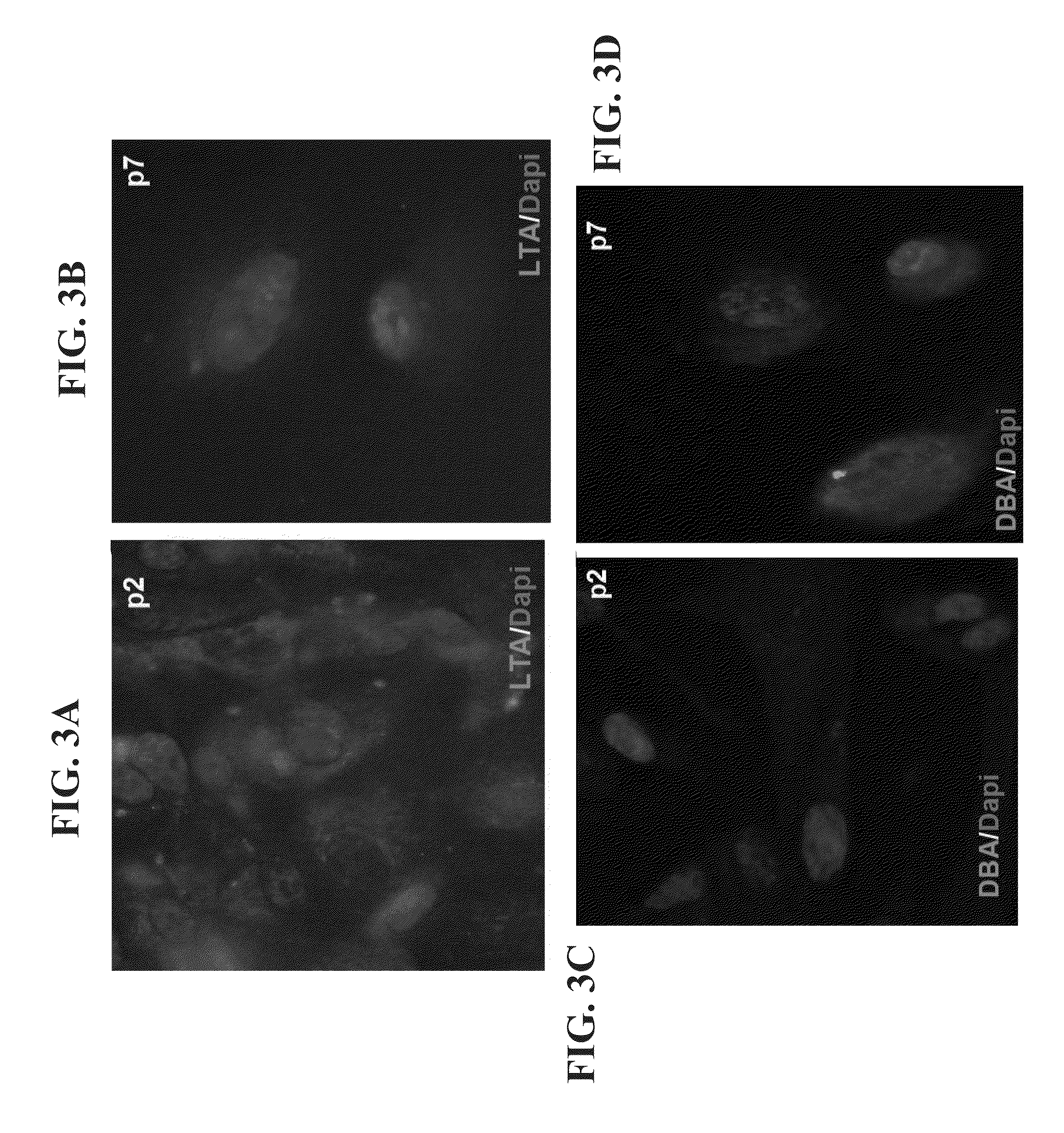
[0199]Following the retrieval of a small specimen of hAK from nephrectomized patients, tissue was dissociated into a single cell suspension and cultured in low densities in T75 flasks so as to allow clonal growth (see scheme in FIG. 1). To achieve expansion to a confluent adherent monlayer culture (P0), low cell numbers were initially grown using either serum containing media (SCM) or defined serum free media (SFM). Cell growth was initiated from small cell foci. However, while both media enabled cell expansion, SFM promoted more concentric, well defined expansion and SCM displayed rapid expansion in a less-organized manner (FIGS. 2A-F). Staining of cultures for segment-specific markers [lotus tetragonolobus (LTA)-proximal tubules; Tamm-Horsfall glycoprotein (THG)-distal tubules; DBA-collecting tubules] revealed the presence of heterogeneous tubule cell types with predominance of proximal (70%) and distal (20%) tubules and to a lesser extent collecting duct...
example 2
Low-Attachment Conditions in Heterogeneous hAK Cultures Promote Formation of ‘Nephrospheroids’
[0202]The present inventors considered that culture conditions that support proliferation of human kidney cells that form spheroids may represent a strategy for isolation of cells with progenitor potential. Accordingly, heterogeneous P0 adherent SCM and SFM cultures originating from five hAK samples were subjected to low attachment conditions—specifically they were seeded on polyHEMA plates at a density of 20-40,000 viable cells / ml.
[0203]After 7-10 days, floating cellular aggregates, termed nephrospheroids or hKEpC spheroids, 100-130 micrometer in diameter, were obtained from 10 of the 10 cases (FIG. 6A). Primary kidney-spheres, once enzymatically disaggregated into single cells and replated at a density of 20,000 cells / ml in ultra-low attachment plates could give rise to secondary spheres within 5-7 days of culture. Having determined that P2 spheroids could be reproducibly generated follow...
example 4
Heterogeneous hAK Cells Cultured in Fetal Kidney Conditioned Media Show Enhanced Clonogenicity
[0215]Following the observation that heterogeneous cultures of kidney epithelial cells maintain the ability to form spheres, the present inventors analyzed culture conditions that enhance cells' clonogenic capacities and would allow for clonal analysis of hAK cell subpopulations. Cells were plated in limiting dilution (LD) concentrations under 4 different growth conditions: a) SCM, b) SFM, c) culture expanded in SCM and LD analysis performed in SFM, d) SCM in 1:1 ratio with fetal kidney conditioned media (FKCM). Analysis of the number of colonized wells, recorded after 4 weeks, showed that SCM promotes higher clonogenic capacities compared to SFM. This was especially evident when combined with FKCM (condition d) (FIGS. 12A-C). FKCM clones showed improved viability and confluence as opposed to SCM (FIGS. 13A-D). Clonogenic expansion indicative of self-renewal could be performed for more than...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com