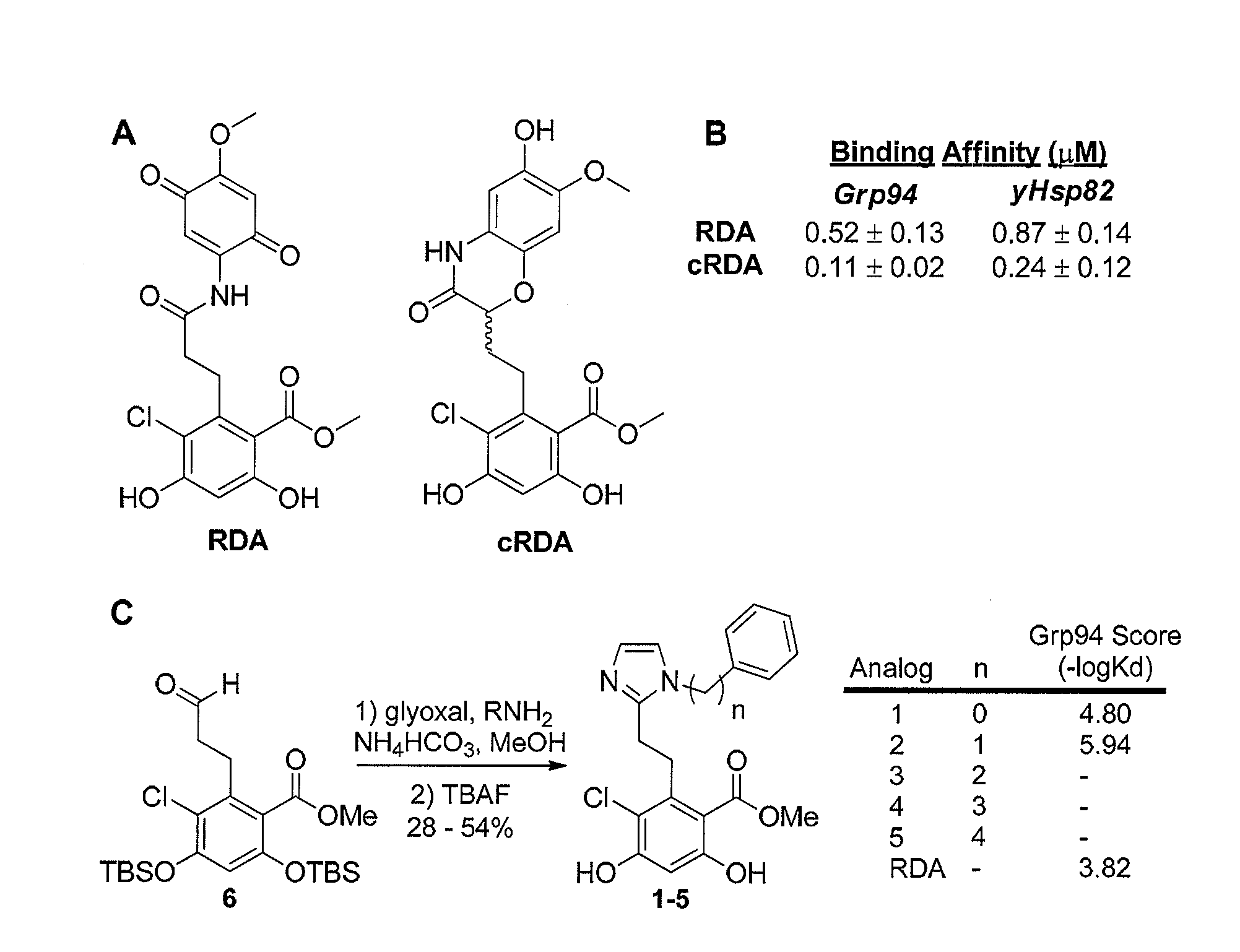
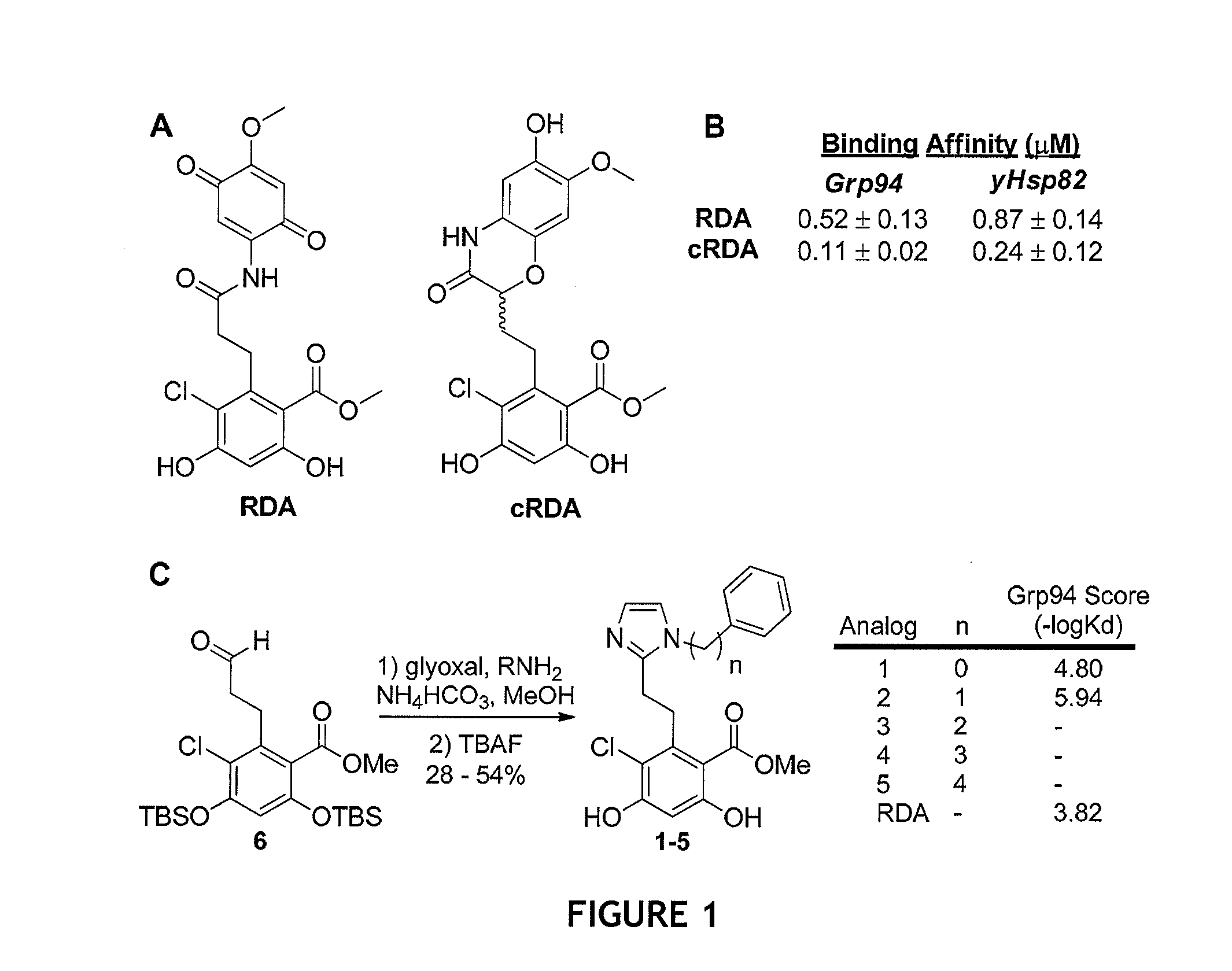
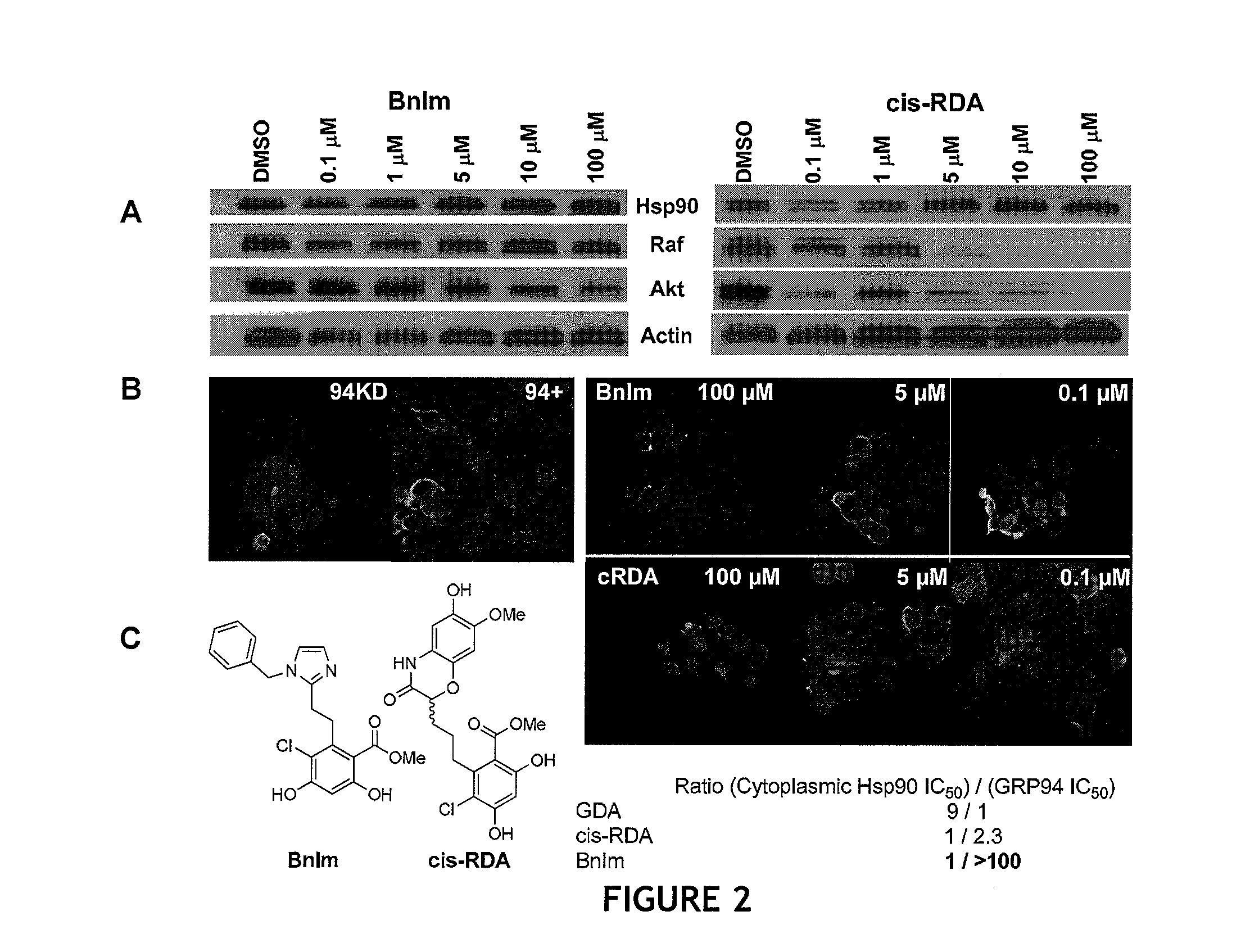
Grp94 inhibitors
a technology of inhibitors and grp94, which is applied in the field of grp94 inhibitors, can solve the problems of non-selective inhibition, no isoform selective inhibitors have yet been discovered, and multiple detriments,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of GRP94 Inhibitors
[0226]General Method for the Synthesis of Compounds 1-5. Aldehyde 6 (1 equiv.) was dissolved in wet MeOH at 25° C. The required aniline / amine (1 equiv.) was added dropwise via a syringe to the reaction flask followed by addition of ammounim bicarbonate (1 equiv.). Glyoxal (1 equiv.) was then added dropwise via a syringe and the reaction was allowed to stir at 25° C. for 8 h. Upon complete conversion of the aldehyde, as observed by thin-layer chromatography, tetrabutylammonium fluoride was added dropwise via syringe and the reaction was allowed to stir at 25° C. for 30 min, at which time, the reaction was quenched with sat. aq. NH4Cl and extracted with EtOAc. The organic layers were combined, dried over Na2SO4, and concentrated in vacuo. All compounds were purified via flash chromatography utilizing 95:5 (CH2Cl2:MeOH) as the eluent. Yields and characterization for all compounds are provided below.
Compound 2, Methyl 2-(2-(1-benzyl-1H-imidazol-2-yl)ethyl)-3...
example 2
Cell Culture
[0243]HEK293 and C2C12 cells were maintained in DMEM supplemented with non-essential amino acids, L-glutamine (2 mM), streptomycin (500 μg / mL), penicillin (100 units / mL), and 10% FBS. Cells were grown to confluence in a humidified atmosphere (37° C., 5% CO2). Stable GRP94-siRNA knockdown cell lines were generated as folllows: the shRNA sequence 5′-GGCUCAAGGACAGAUGAUGtt-3′ was cloned into the A pSilencer 2.0-U6 vector (Ambion) and positive clones confirmed by sequencing. The pSilencer 2.0-U6-GRP94 siRNA vector and a control, non-targeting pSilencer 2.0-U6 siRNA vector (scrambled, control) were transfected into HEK293 cells using Lipofectamine 2000 and the manufacturers protocol. Cell cultures were selected thirty six hours post-transfection by addition of 1 microgram / ml puromycin to the media. Puromycin resistant clones (both GRP94 siRNA and non-targeting siRNA) were subsequently expanded and screened for knockdown efficiency by immunoblotting, using GRP94 antibody DU120....
example 3
Toll Trafficking Assay Protocol
[0244]Prior studies by Nicchitta have shown that the Toll receptors are solely dependent upon GRP94 for their trafficking to the cell membrane, as illustrated in FIG. 4. Thus, they have used surface expression of Toll to monitor GRP94-mediated trafficking, which in the presence of siRNA targeting GRP94, little to no Toll is presented at the cell surface. HEK293 cells stably expressing a GRP94 siRNA or scrambled siRNA are transfected with a plasmid encoding the expression of TOLL, the drosophila homologue of the human unterleukin 1 receptor. After transfection, cells are treated with Hsp90 inhibitors for 24 hours. After drug tteatment the surface expression of Toll is monitored by either fluorescence microscopy or flow cytometry. The trafficking of Toll to the cell surface is dependent on GRP94. Cells deficient in GRP94 (i.e. GRP94 siRNA) or cells that have been treated with GRP94 inhibitor will show reduced expression of Toll. Western blots are then us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com