Methods and compositions for preserving photoreceptor and retinal pigment epithelial cells
a technology of epithelial cells and photoreceptors, applied in the direction of drug compositions, enzyme inhibitors, peptide/protein ingredients, etc., can solve the problems of z-vad, a pan-caspase inhibitor, failing to prevent photoreceptor loss, etc., to reduce or prevent the loss of photoreceptors and/or retinal pigment epithelial cells, reduce the loss of visual function, and prevent the loss of photoreceptors and/or retinal pigmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
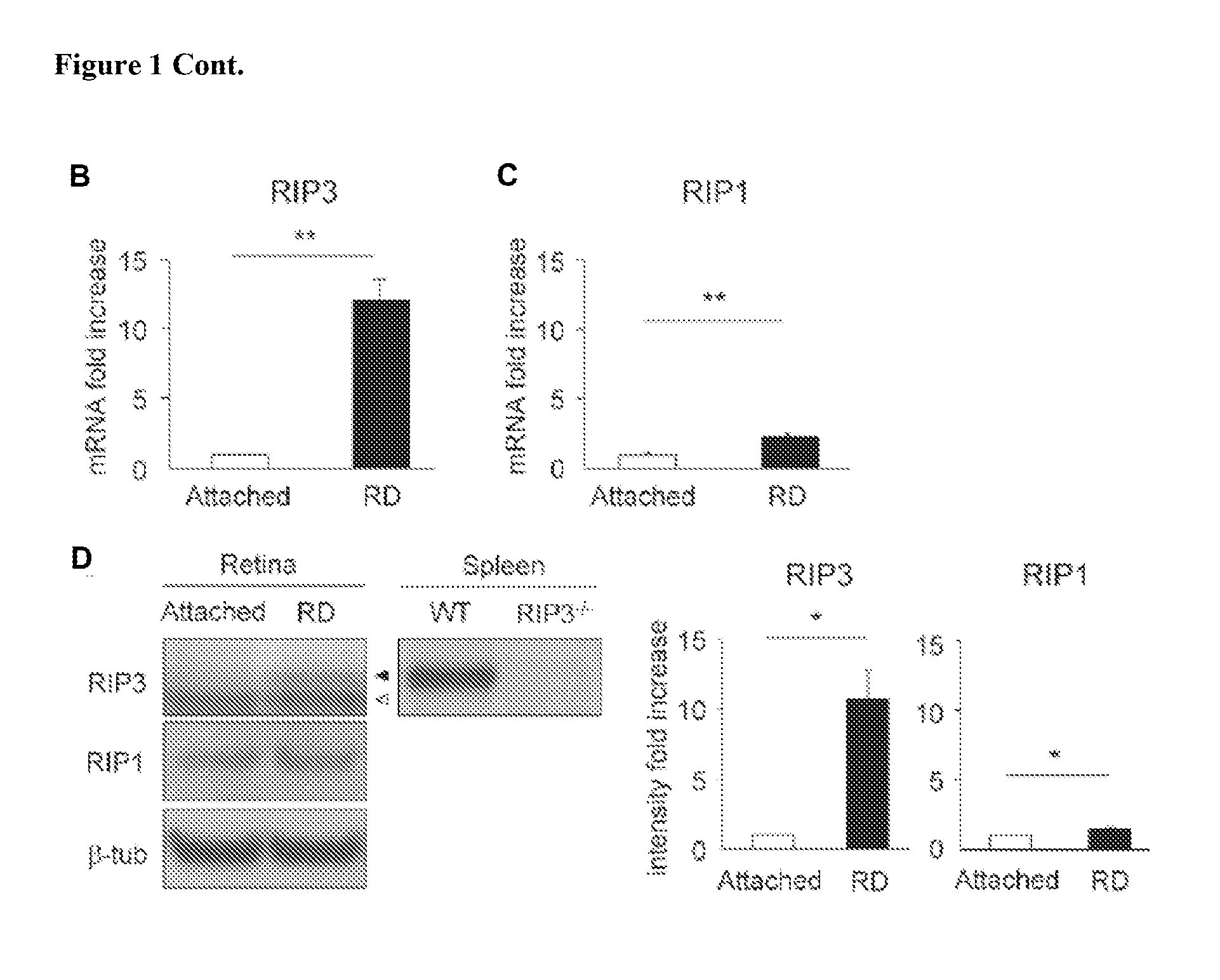
Increased Expression of RIP-3 and RIP-1 after Retinal Detachment
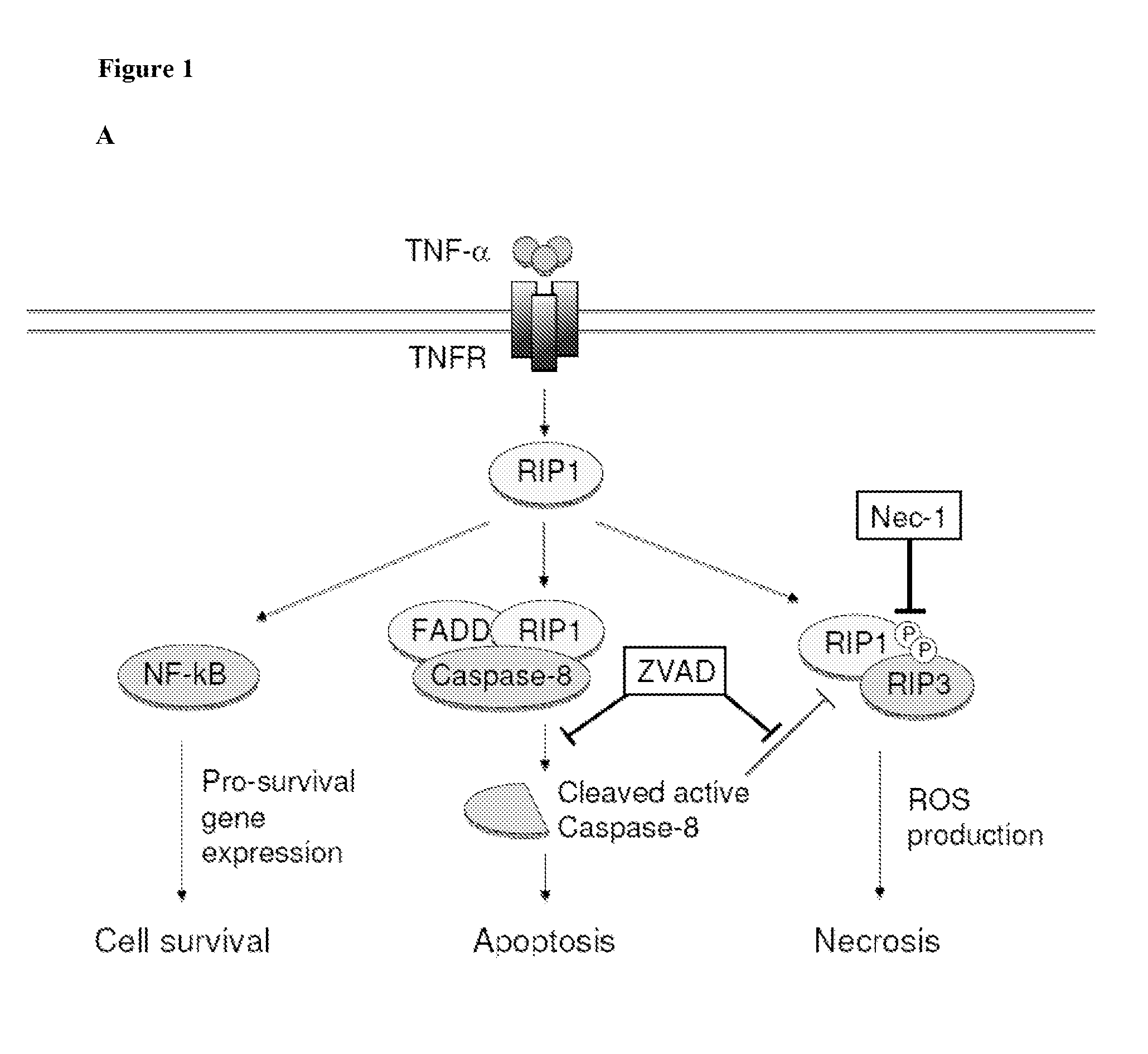
[0384]Photoreceptor death after retinal detachment has been thought to be caused mainly by apoptosis (Cook et al. (1995) INVEST OPHTHALMOL VIS SCI 36:990-996; Arroyo et al. (2005) AM J OPHTHALMOL 139:605-610. Although caspase-dependent pathway is known to be activated after retinal detachment, caspase inhibition by pan-caspase inhibitor fails to prevent photoreceptor death (Zacks et al. (2003) INVEST OPHTHALMOL VIS SCI 44:1262-1267; Hisatomi et al. (2001) AM J OPHTHALMOL 158:1271-1278). In this example, other pathways leading to photoreceptor death were investigated. In particular, the expression levels of RIP-1 and RIP-3 proteins were measured after retinal detachment. RIP-3 is a key regulator of RIP-1 kinase activation (Galluzzi L et al. (2009) J. MOL. CELL BIOL.) and its expression level has been shown to correlate with responsiveness to programmed necrosis (He S et al. (2009) CELL 137:1100-1111). As described below,...
example 2
Necrosis Inhibitor Prevents Retinal Detachment-Induced Photoreceptor Death in Combination with a Caspase Inhibitor
[0393]Necrostatin-1 (Nec-1) is a potent and selective inhibitor of programmed necrosis, which targets RIP-1 kinase activity (Degterev A et al. (2008) NAT. CHEM. BIOL. 4:313-321). The effectiveness of a RIP-1 kinase inhibitor in combination with a capsase inhibitor to prevent retinal detachment induced photoreceptor death was investigated.
[0394]RIP-1 is phosphorylated at several serine residues in its kinase domain during programmed necrosis and Nec-1 inhibits this phosphorylation (Degterev (2008) supra; Cho Y et al. (2009) Cell 137:1100-1111). To investigate the role of RIP-1 kinase in retinal detachment-induced photoreceptor death, the phosphorylation status of RIP-1 was first assessed by immunoprecipitating RIP-1 from the retina (FIG. 3A) and then blotting with an anti-phosphoserine antibody. RIP-1 was immunoprecipitated from retinal lysates. One mg of retinal lysates ...
example 3
Caspase Inhibition Shifts Retinal Detachment-Induced Photoreceptor Death from Apoptosis to Programmed Necrosis
[0402]The morphology of photoreceptors after retinal detachment was examined by transmission electron microscopy (TEM) and propidium iodide (PI) staining and the effect of treatment with Z-VAD and / or a Nec-1 compound of Formula I-C was analyzed.
[0403]TEM was performed as previously described (Hisatomi T et al. (2008). J. CLIN. INVEST. 118:2025-2038). The specimens were observed with Philips CM10 electron microscope. Over 200 photoreceptors each eye were photographed and subjected to quantification of cell death modes in a masked fashion. Photoreceptors showing cellular shrinkage and nuclear condensation were defined as apoptotic cells, while photoreceptors associated with cellular and organelle swelling and discontinuities in nuclear and plasma membrane were defined as necrotic cells. The presence of electron-dense granular materials were reported to occur subsequent to both...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com