Oral treatment of inflammatory bowel disease
a technology for inflammatory bowel disease and oral treatment, which is applied in the direction of biocide, drug composition, peptide/protein ingredients, etc., can solve the problems of inflamed intestine blood loss, abdominal pain, diarrhea, rectal bleeding, etc., and achieves the effects of reducing the risk of inflammatory bowel diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
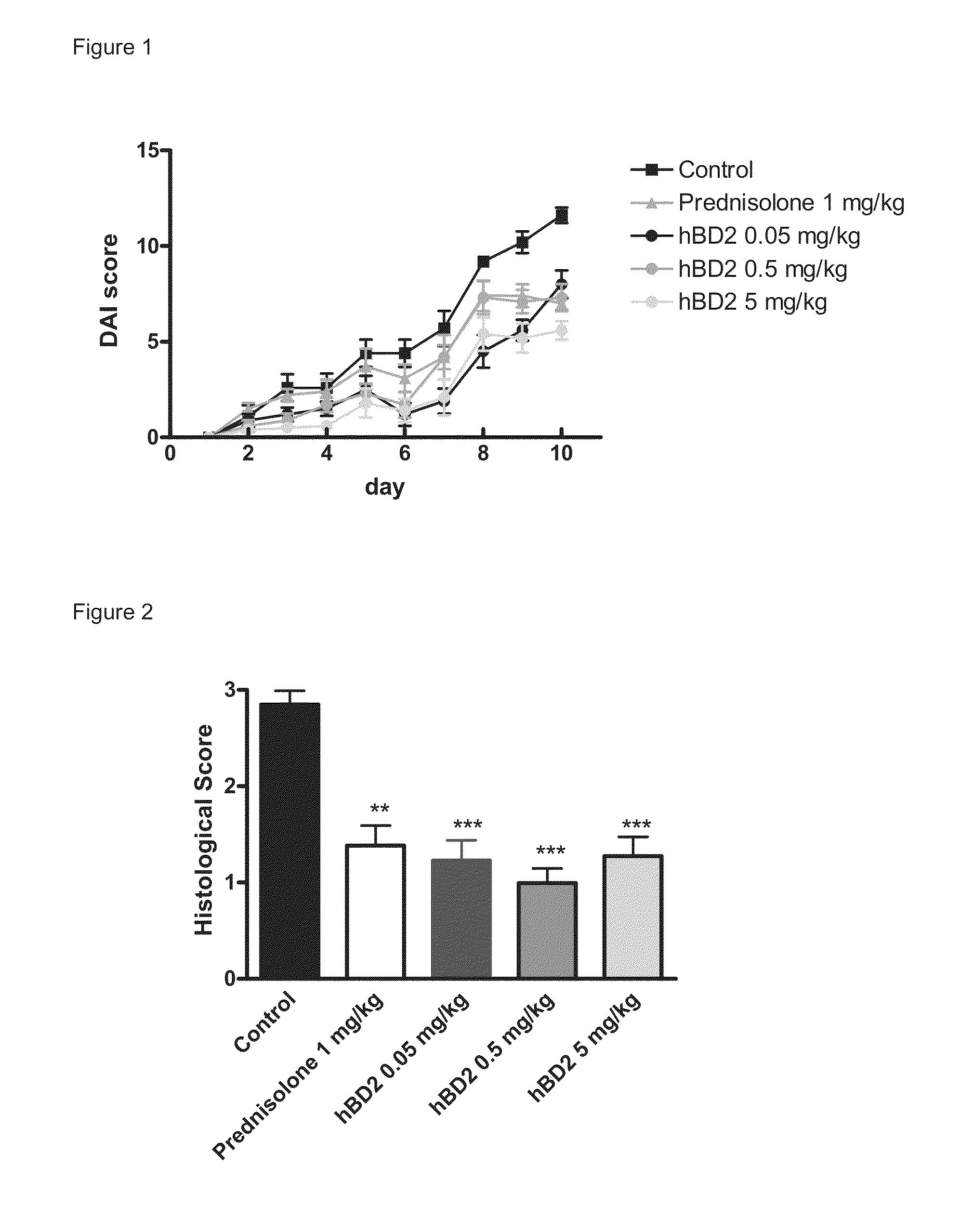
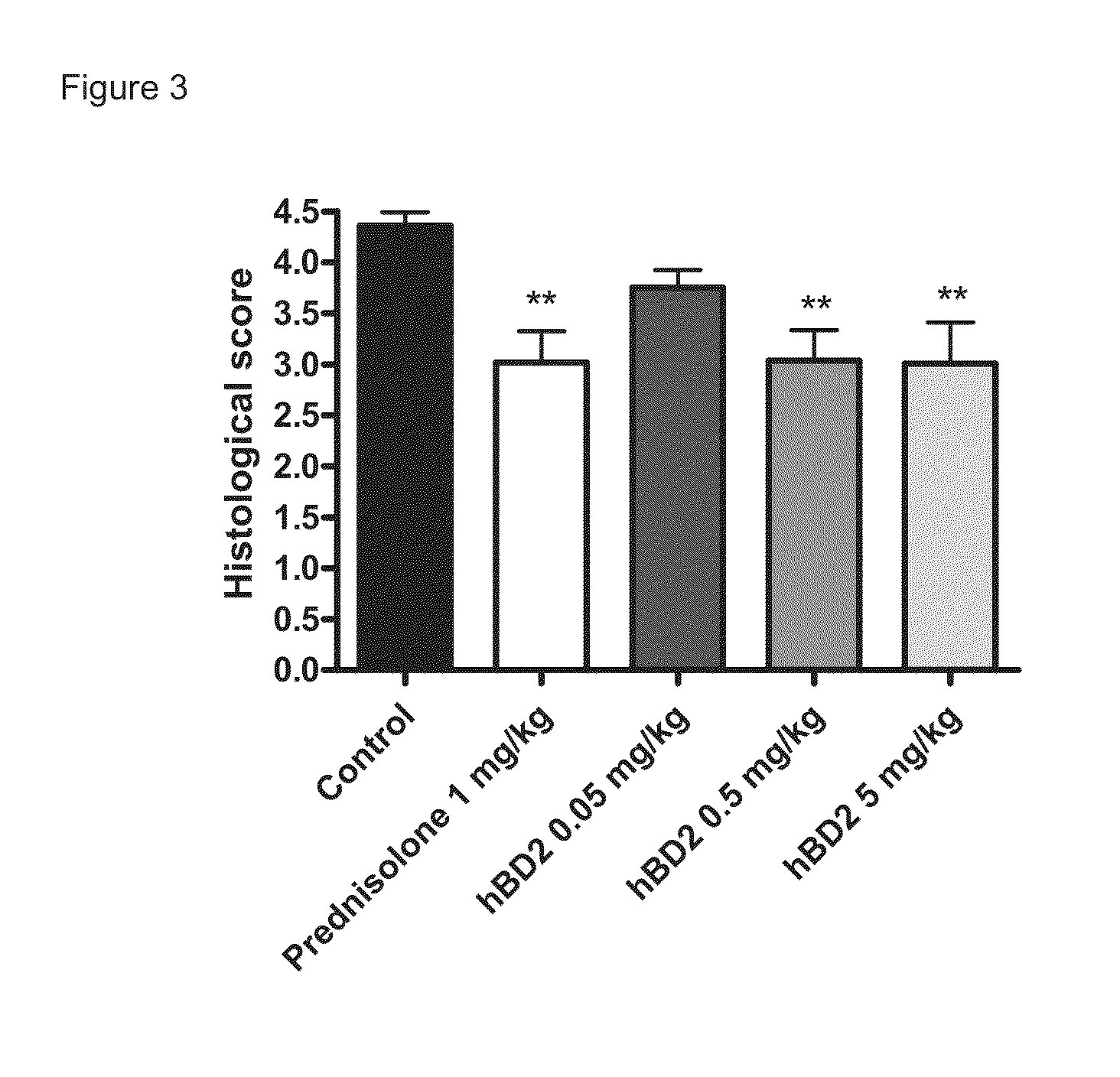
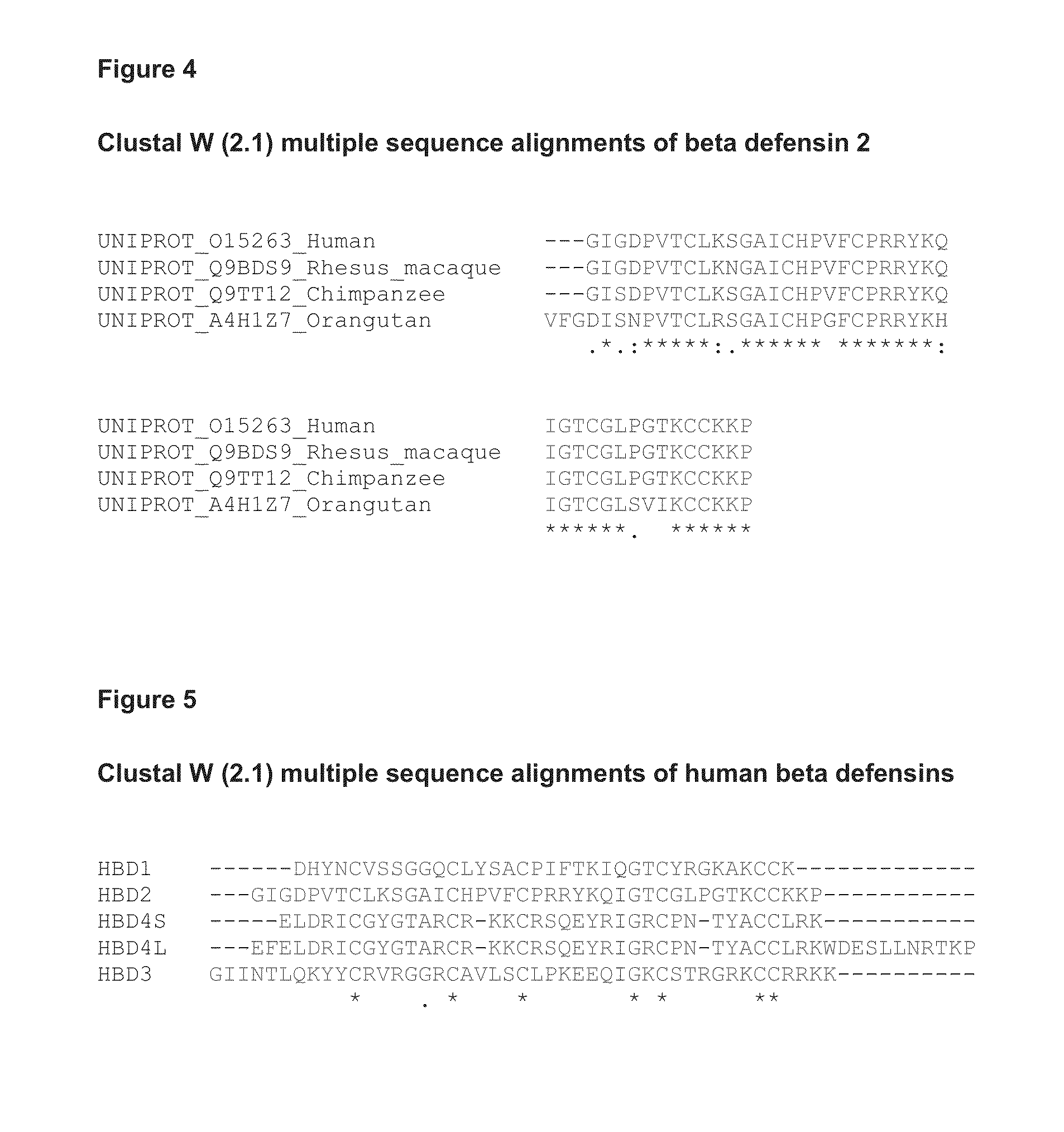
[0114]10-Day Dextran Sodium Sulphate (DSS)-Induced Colitis Model in the Mouse
[0115]The aim of the following study was to determine the anti-inflammatory activity of human beta defensin 2 in an acute (10-days) model of inflammatory bowel disease (colitis) induced by oral dextran sodium sulphate (DSS) administration in the mouse.
[0116]The DSS colitis mouse model is a well recognized model for studying inflammatory bowel disease, as described in Kawada et al. “Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease”, World J. Gastroenterol., Vol. 13 (42), pp. 5581-5593 (2007); and Wirtz and Neurath “Mouse models of inflammatory bowel disease”, Advanced Drug Delivery Reviews, Vol. 59 (11), 1073-1083 (2007).
[0117]Materials
[0118]Test Items
[0119]Human beta defensin 2 (hBD2); Methylprednisolone 21-hemisuccinate (“prednisolone”), PBS buffer (GIBCO).
[0120]Experimental Animals
[0121]Male C57BL / 6 mice (Harlan Interfauna Iberica, Barcelona,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com