Engineered tissues for in vitro research uses, arrays thereof, and methods of making the same
a technology of in vitro research and tissue, applied in the field of in vitro research and development cost of a new pharmaceutical compound, can solve the problems of low rate of new therapeutic discovery, unsustainable r&d cost, and long drug discovery process, so as to increase the number and quality of innovative, cost-effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cell Culture
[0188]Smooth Muscle Cells
[0189]Primary human aortic smooth muscle cells (HASMC; GIBCO / Invitrogen Corp., Carlsbad, Calif.) were maintained and expanded in low glucose dulbecco's modified eagle medium (DMEM; Invitrogen Corp., Carlsbad, Calif.) supplemented with 10% fetal bovine serum (FBS), 100 U / mL Penicillin, 0.1 mg / mL streptomycin, 0.25 μg / mL of amphotericin B, 0.01M of HEPES (all from Invitrogen Corp., Carlsbad, Calif.), 50 mg / L of proline, 50 mg / L of glycine, 20 mg / L of alanine, 50 mg / L of ascorbic acid, and 3 μg / L of CuSO4 (all from Sigma, St. Louis, Mo.) at 37° C. and 5% CO2. Confluent cultures of HASMC between passage 4 and 8 were used in all studies.
[0190]Endothelial Cells
[0191]Primary human aortic endothelial cells (HAEC; GIBCO / Invitrogen Corp., Carlsbad, Calif.) were maintained and expanded in Medium 199 (Invitrogen Corp., Carlsbad, Calif.) supplemented with 10% FBS, 1 μg / mL of hydrocortisone, 10 ng / mL of human epidermal growth factor, 3 ng / mL of basic fibroblas...
example 2
NovoGel™ Solutions and Mold
[0200]Preparation of 2% and 4% (w / v) NovoGel™ Solution
[0201]1 g or 2 g (for 2% or 4% respectively) of NovoGel™ (Organovo, San Diego, Calif.) was dissolved in 50 mL of Dulbecco's phosphate buffered saline (DPBS; Invitrogen Corp., Carlsbad, Calif.). Briefly, the DPBS and NovoGel™ are heated to 85° C. on a hot plate with constant stirring until the NovoGel™ dissolves completely. NovoGel™ solution is sterilized by steam sterilization at 125° C. for 25 minutes. The NovoGel™ solution remains in liquid phase as long as the temperature is maintained above 65.5° C. Below this temperature a phase transition occurs, the viscosity of the NovoGel™ solution increases and the NovoGel™ forms a solid gel.
[0202]Preparation of NovoGel™ Mold
[0203]An NovoGel™ mold was fabricated for the incubation of cylindrical bio-ink using a Teflon® mold that fit a 10 cm Petri dish. Briefly, the Teflon® mold was pre-sterilized using 70% ethanol solution and subjecting the mold to UV light f...
example 3
Fabrication of HASMC-HAEC Polytypic Cylindrical Bio-Ink
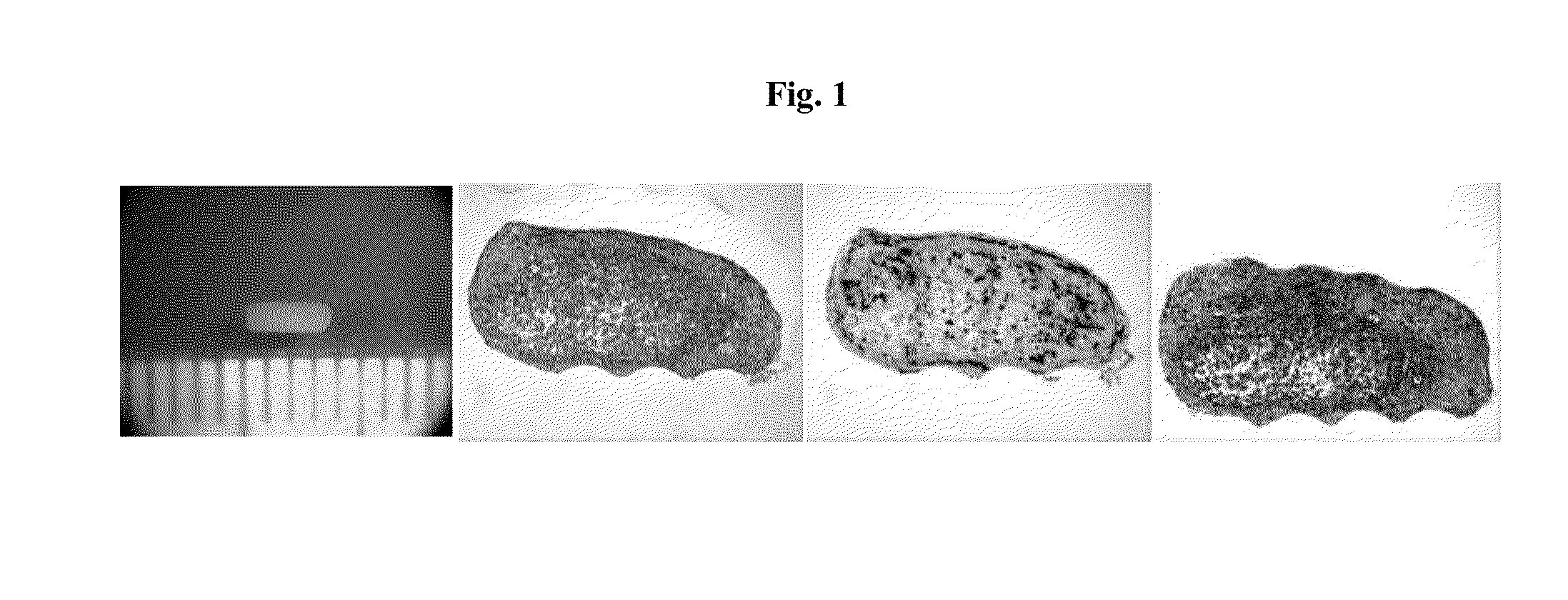
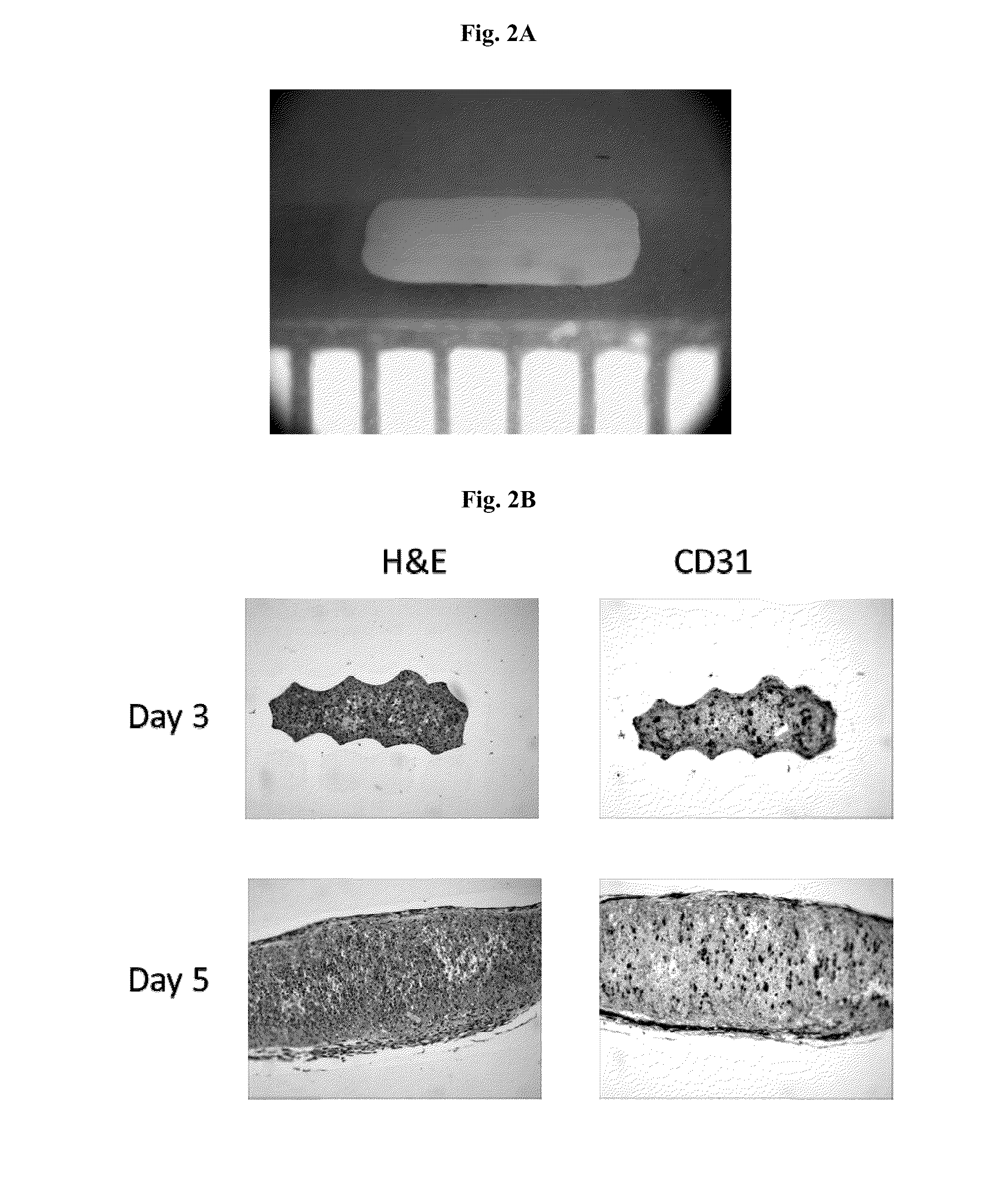
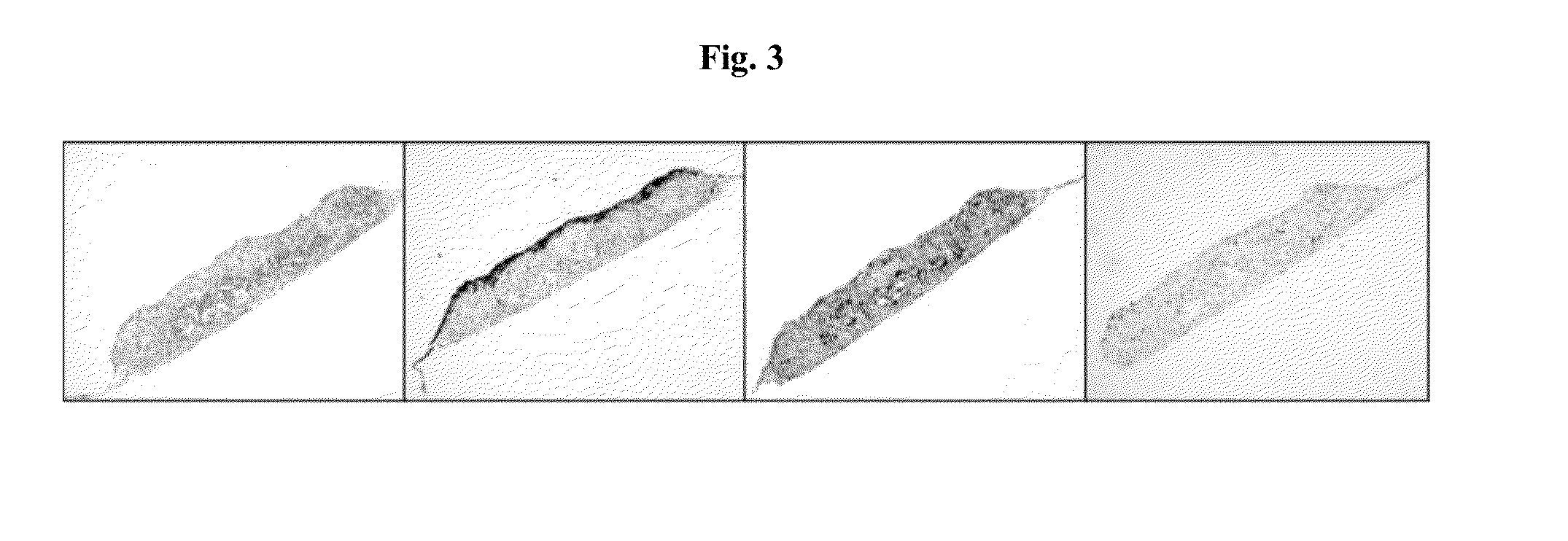
[0204]To prepare polytypic cylindrical bio-ink, HASMC and HAEC were individually collected and then mixed at pre-determined ratios. Briefly, the culture medium was removed from confluent culture flasks and the cells were washed with DPBS (1 ml / 5 cm2 of growth area). Cells were detached from the surface of the culture flasks by incubation in the presence of trypsin (1 ml / 15 cm2 of growth area; Invitrogen Corp., Carlsbad, Calif.) for 10 minutes. HASMC were detached using 0.15% trypsin while HAEC were detached using 0.1% trypsin. Following the incubation appropriate culture medium was added to the flasks (2× volume with respect to trypsin volume). The cell suspension was centrifuged at 200 g for 6 minutes followed by complete removal of supernatant solution. Cell pellets were resuspended in respective culture medium and counted using a hemocytometer. Appropriate volumes of HASMC and HAEC were combined to yield a polytypic cell susp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| internal diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com