Electrode material and method for producing the same
a technology of electrodes and materials, applied in the field of electrode materials, can solve the problems of low conductivity, reduce internal resistance, uneven conductivity, etc., and achieve the effects of uneven conductivity, uneven conductivity, and uneven conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
[0039]An embodiment (first embodiment) of an electrode material and a method for producing the same of the invention will be described.
[0040]In addition, this embodiment makes a description in detail for easy comprehension of the gist of the invention, and does not limit the invention unless otherwise stated.
[0041][Electrode Material]
[0042]An electrode material of this embodiment includes an aggregate formed by aggregating electrode-active material particles having a carbonaceous film formed on a surface, an average particle size of the aggregate is 0.5 to 100 μm, and a volume density of the aggregate is 50 to 80 vol % of the volume density in a case in which the aggregate is a solid.
[0043]Here, it is assumed that a solid aggregate is an aggregate in which a void is not present at all, and a density of an actual aggregate is the same as a theoretical density of an electrode-active material.
[0044]Here, the aggregate that is formed by aggregating each electrode-active material having ...
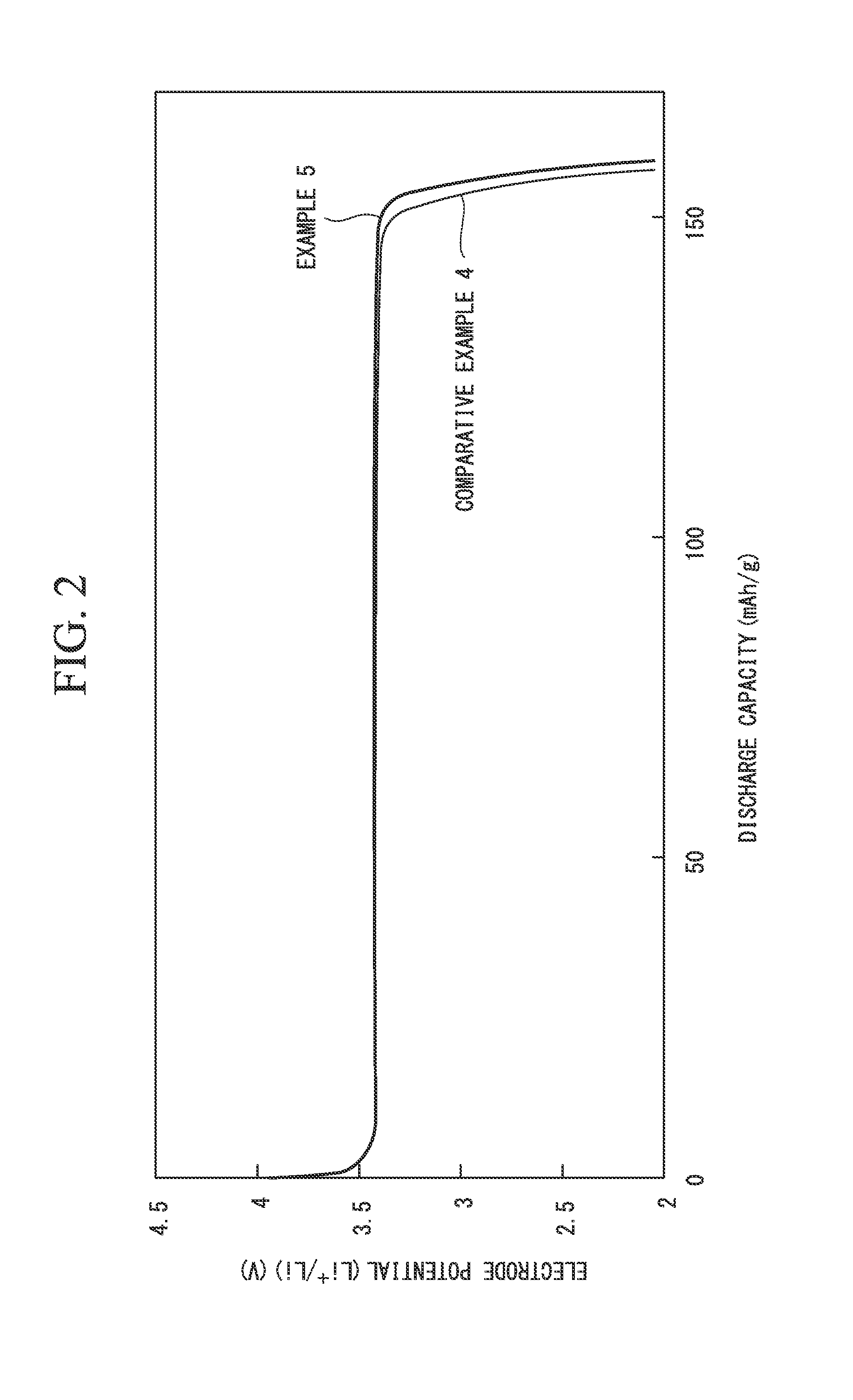
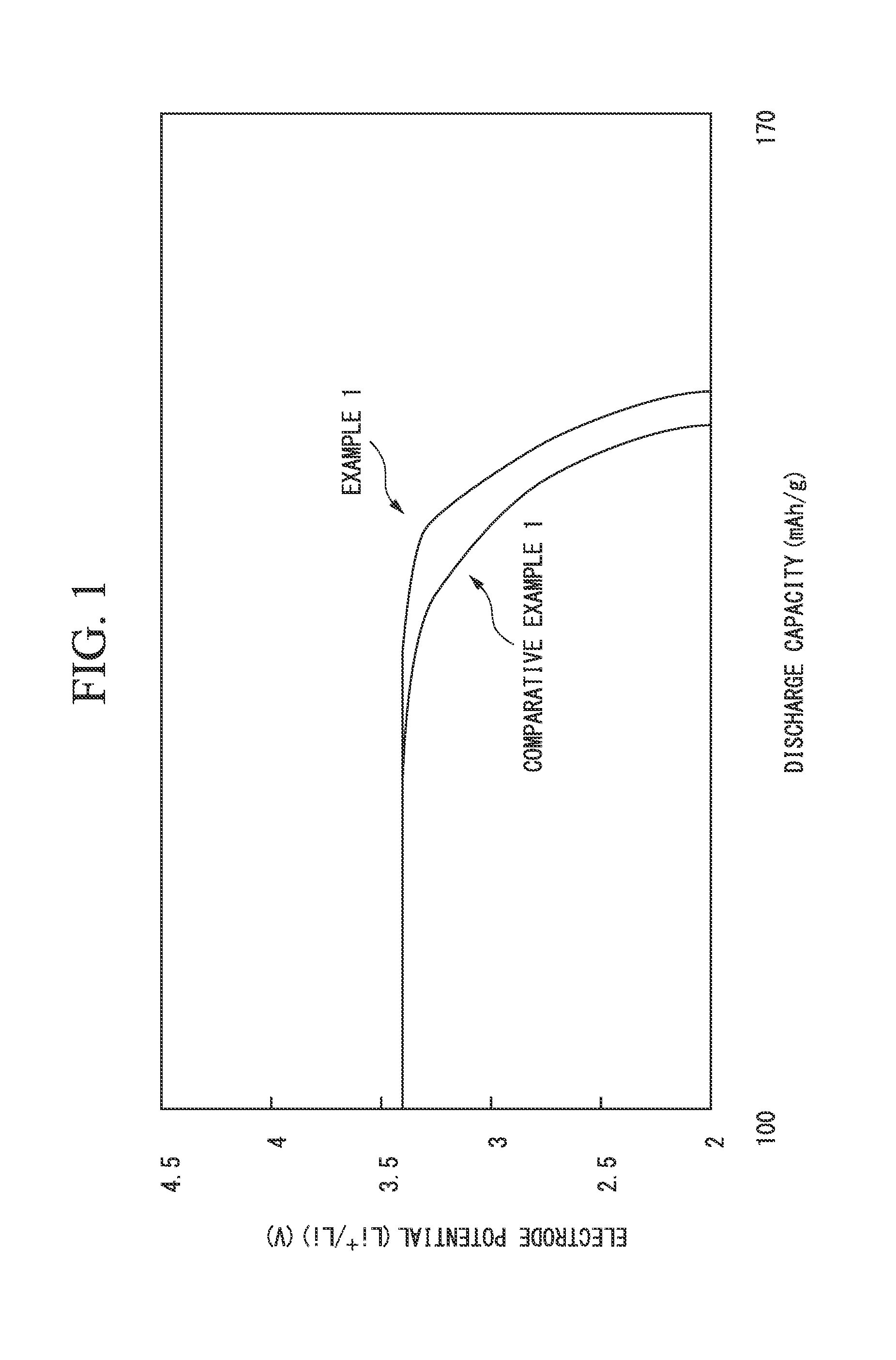
example 1
[0099](Preparation of Electrode Material)
[0100]4 mol lithium acetate (LiCH3COO), 2 mol iron (II) sulfate (FeSO4), and 2 mol phosphoric acid (H3PO4) were mixed to 2 L (liters) of water so that the entire amount became 4 L, whereby a uniform slurry mixture was prepared.
[0101]Then, this mixture was accommodated in an 8-L pressure-resistant airtight container, and hydrothermal synthesis was performed at 120° C. for one hour.
[0102]Then, a precipitate that was obtained was washed with water, whereby a cake-shaped precursor of the electrode-active material was obtained.
[0103]Then, 150 g (in terms of a solid content) of the precursor of the electrode-active material, an aqueous polyvinyl alcohol solution, which was obtained dissolving 20 g of polyvinyl alcohol in 200 g of water, as the organic compound, and 500 g of zirconia balls having a diameter of 5 mm as a medium particle were put into a ball mill, and a dispersion treatment was carried out after adjusting a stirring time of the ball m...
example 2
[0135]An electrode material, and a positive electrode of the lithium ion battery were prepared in the same manner as Example 1 except that the stirring time of the ball mill was adjusted so that D90 / D10 of the particle size distribution of the precursor particle of the electrode-active material in the slurry became 10. Then, evaluation was performed. Evaluation results are shown in Table 1.
[0136]In addition, in Example 2, the same voltage drop at the final stage of discharge as Example 1 was also recognized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com