Agents for treating disorders involving modulation of ryanodine receptors
a technology of ryanodine receptor and agent, which is applied in the field of agents for treating disorders involving modulation of ryanodine receptor, can solve the problems of limited therapeutic utility of drugs, and achieve the effect of modulating the amount of ryanodine bound calstabin and preventing or treating a leakage in ryr
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis
Instruments:
[0107]NMR: Bruker AVANCE III 400 or Varian Mercury 300
[0108]LC / MS: Waters Delta 600 equipped with Autosampler 717Plus, Photo Diode Array Detector 2996, and Mass Detector 3100, or Shimadzu 210
General procedure for the alkylation of 7-methoxy-2,3,4,5-tetrahydrobenzo[f][1,4]thiazepine (“Amine”).
[0109]Amine (structure shown above) (1 mmol) was dissolved in 3 ml dichloromethane. To the solution was added alkylation reagent (1 mmol), followed by N,N-diisopropylethylamine (0.34 ml, 2 mmol). The mixture was stirred at room temperature overnight. The solution was loaded onto column directly and eluted with hexane / EtOAc (2:1, v / v).
Compound 2
[0110]Methyl 3-((7-methoxy-2,3-dihydrobenzo[f][1,4]thiazepin-4(5H)-yl)methyl)benzoate. 1HNMR (300 MHz, CDCl3): 7.96 (m, 2H), 7.46 (m, 3H), 6.70 (dd, J=8.4 Hz, 3.0 Hz, 1H), 6.50 (d, J=2.7 Hz, 1H), 4.09 (s, 2H), 3.90 (s, 3H), 3.72 (s, 3H), 3.57 (s, 2H), 3.35 (m, 2H), 2.72 (m, 2H). MS: 344(M+1)
Compound 3
[0111]Methyl 4-((7-methoxy-2,3-dihy...
example 2
Binding of Calstabin2 to PKA-Phosphorylated RyR2
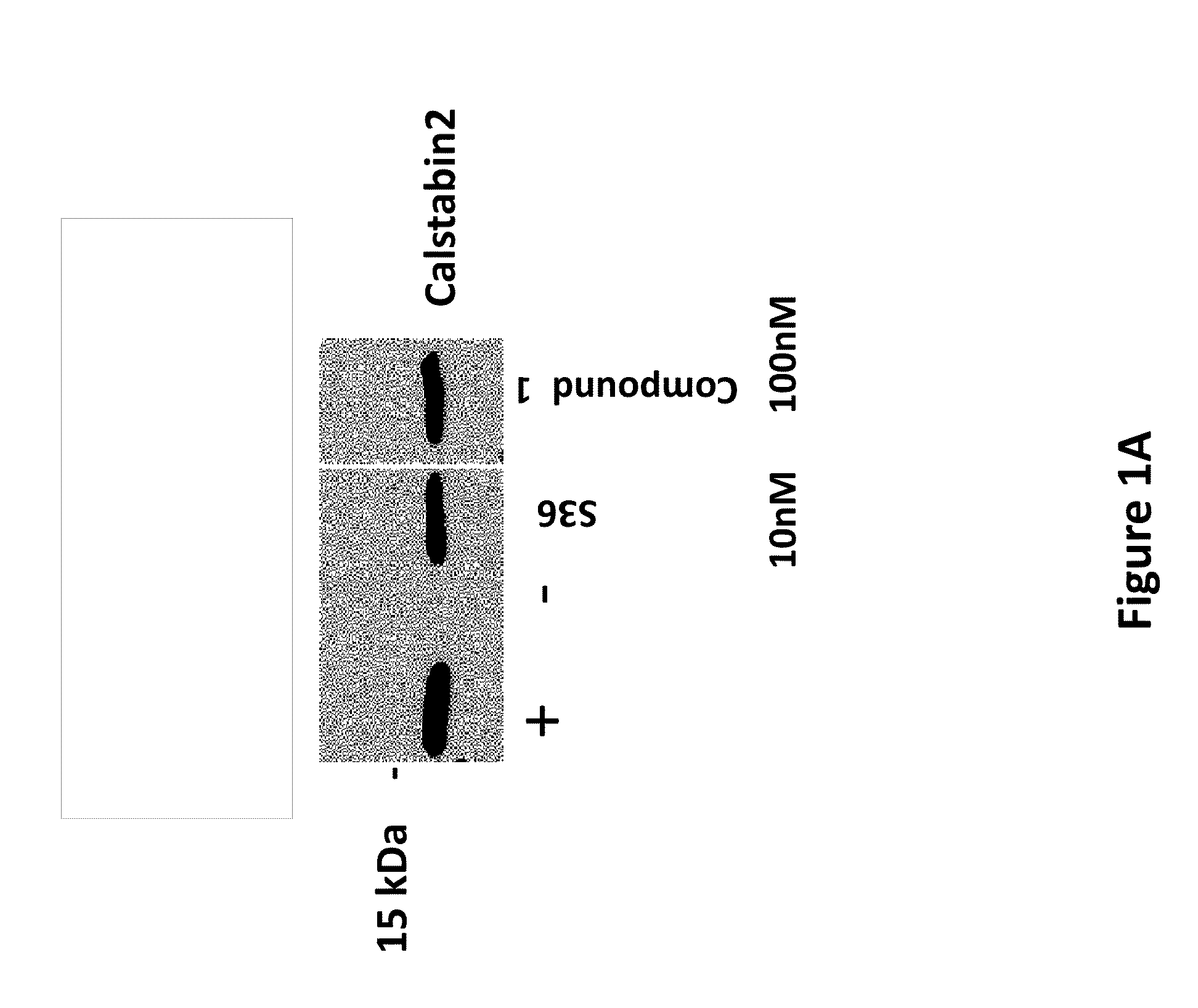
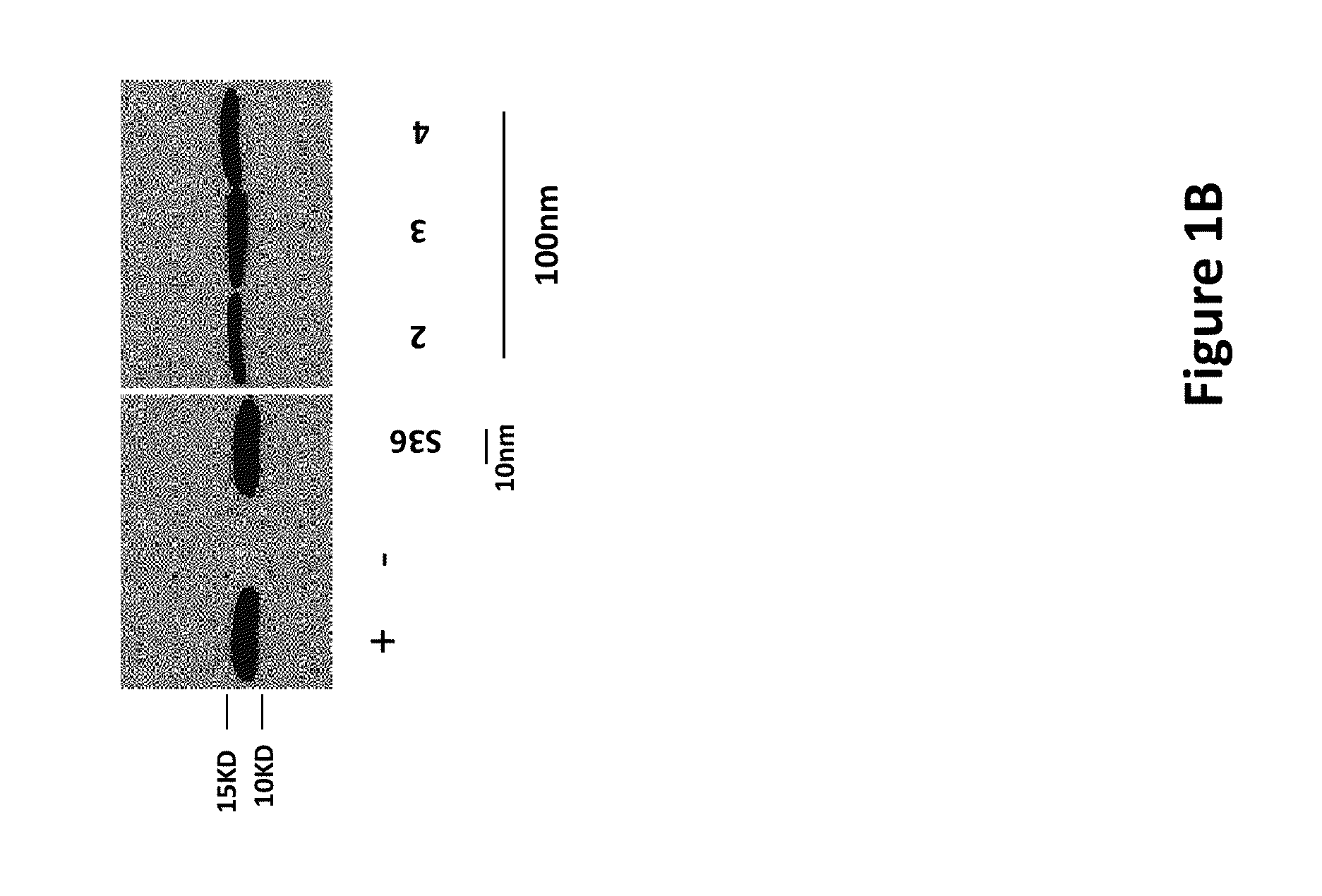
[0141]Cardiac SR membranes were prepared as previously described (Marx et al., 2000; Kaftan et al., Circ. Res., 1996, 78:990-97). Immunoblotting of microsomes (50 μg) was performed as described, with anti-calstabin antibody (1:1,000) (Jayaraman et al., J. Biol. Chem., 1992, 267:9474-77) for 1 hr at room temperature (Reiken et al., Circulation, 107:2459-66, 2003). After incubation with HRP-labeled anti-rabbit IgG (1:5,000 dilution; Transduction Laboratories, Lexington, Ky.), the blots were developed using ECL (Amersham Pharmacia, Piscataway, N.J.) and detected on x-ray film, or exposed to secondary antibodies labeled with infrared Dye and visualized on equipment from Li-Cor Biosciences (model Odyssey). Unless otherwise stated, compounds were tested at a concentration of 100 nM. A representative calstabin2 binding assay is presented below.
[0142]A. PKA phosphorylation of cardiac sarcoplasmic reticulum (CSR)
[0143]B. Reaction mixture was se...
example 3
Binding of Calstabin1 to PKA-Phosphorylated RyR1
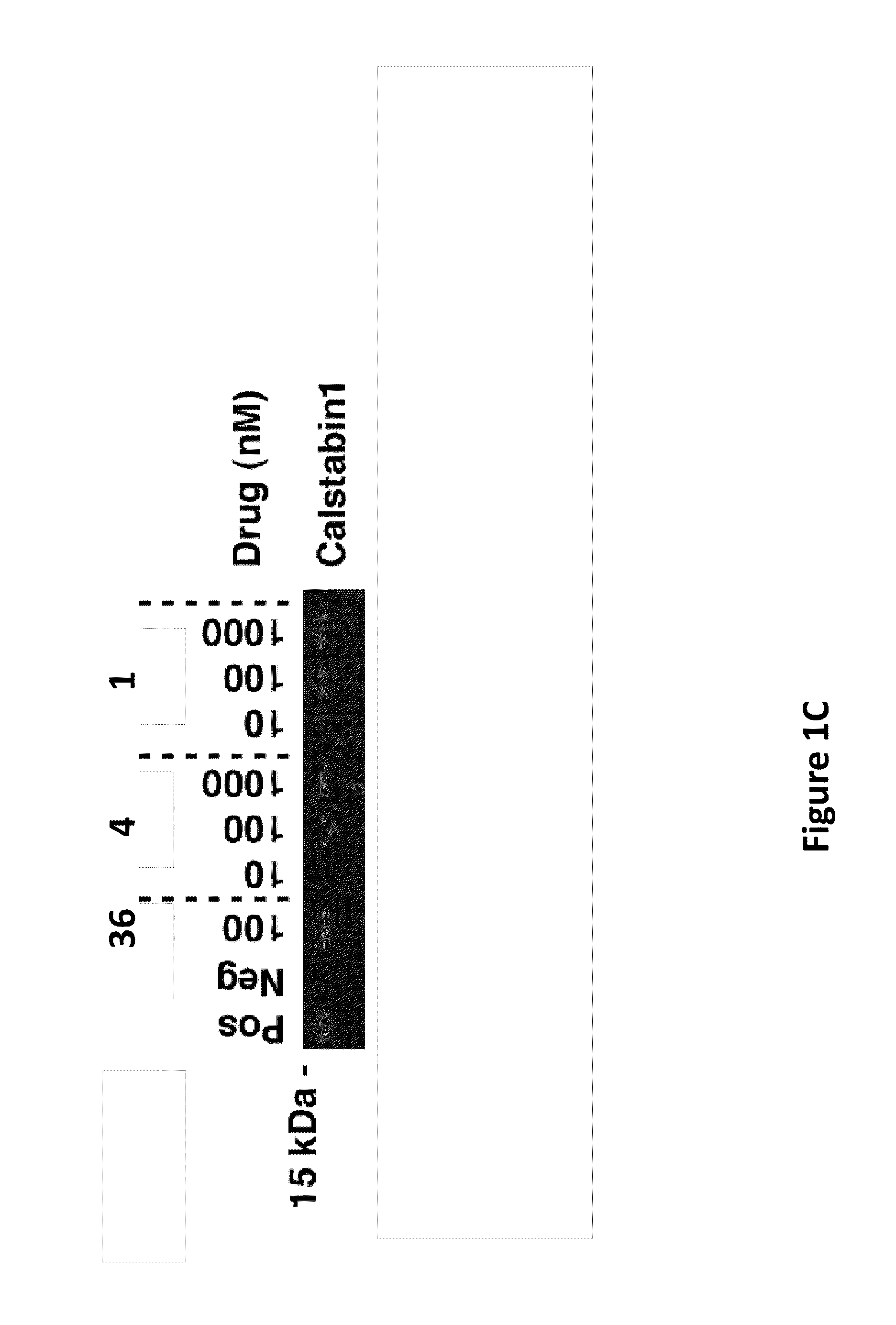
[0173]SR membranes from skeletal muscle were prepared in a manner similar to Example 2, and as further described in US patent application publication No. 2004 / 0224368, the contents of which are incorporated by reference herein. Immunoblotting of microsomes (50 μg) was performed as described, with anti-calstabin1 antibody (Zymed) (1:1,000). The blots were developed and quantified as described in Example 2.
[0174]FIG. 1C depicts an immunoblot with calstabin1 antibody showing binding of calstabin1 to PKA phosphorylated RyR1 in the absence (Neg) or presence of the indicated concentrations of compound 1 or compound 4. (Pos): calstabin binding to non-PKA phosphorylated RyR1. S36 is used as a control. As shown, compound 1 and compound 4 prevented the dissociation of calstabin1 from PKA phosphorylated RyR1 and / or enhanced the (re)binding of calstabin1 to PKA-phosphorylated RyR1 in a dose-dependent manner, with an estimated EC50 of about 100 nM ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| PKA | aaaaa | aaaaa |
| PKA | aaaaa | aaaaa |
| frequencies | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com