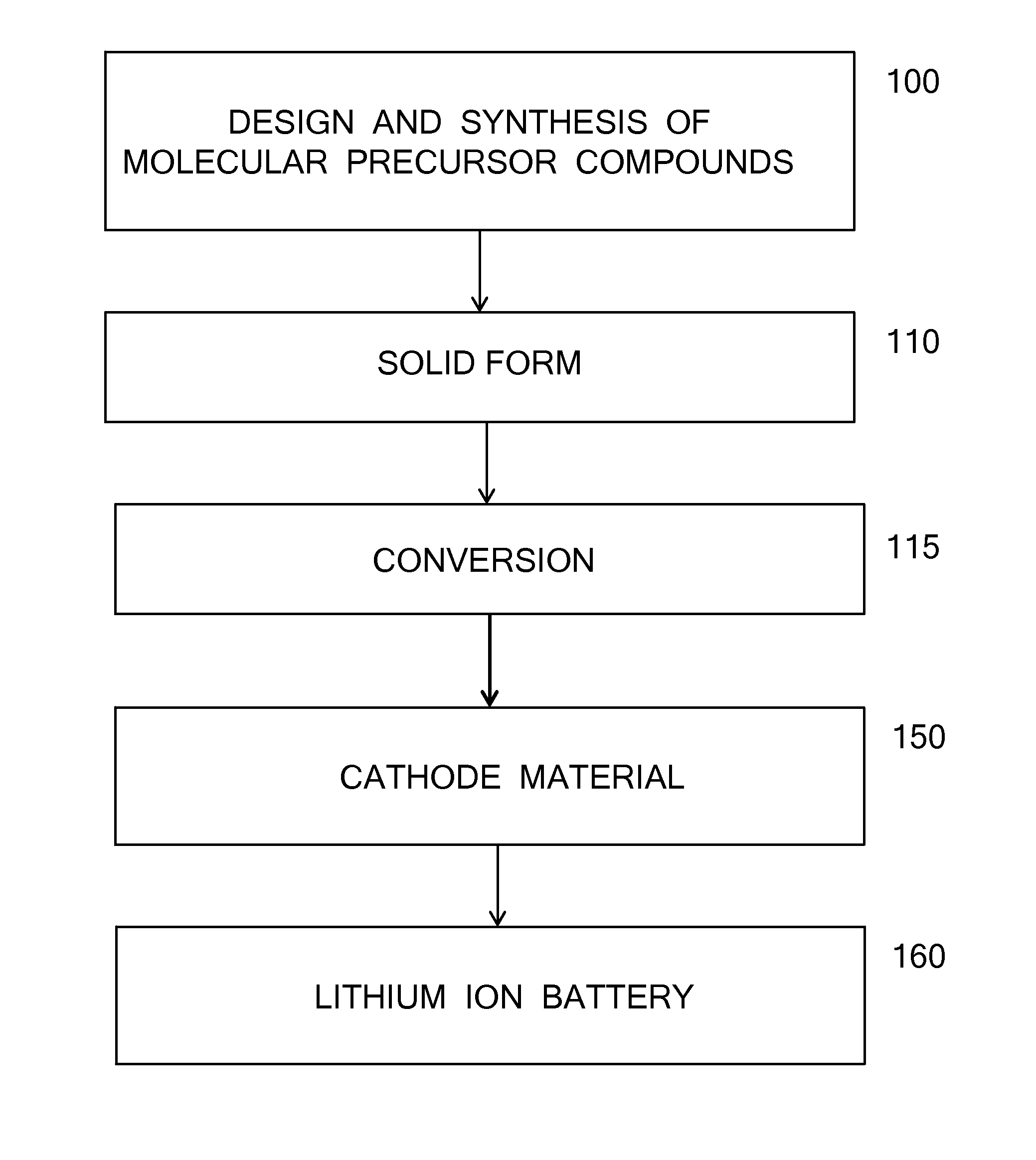
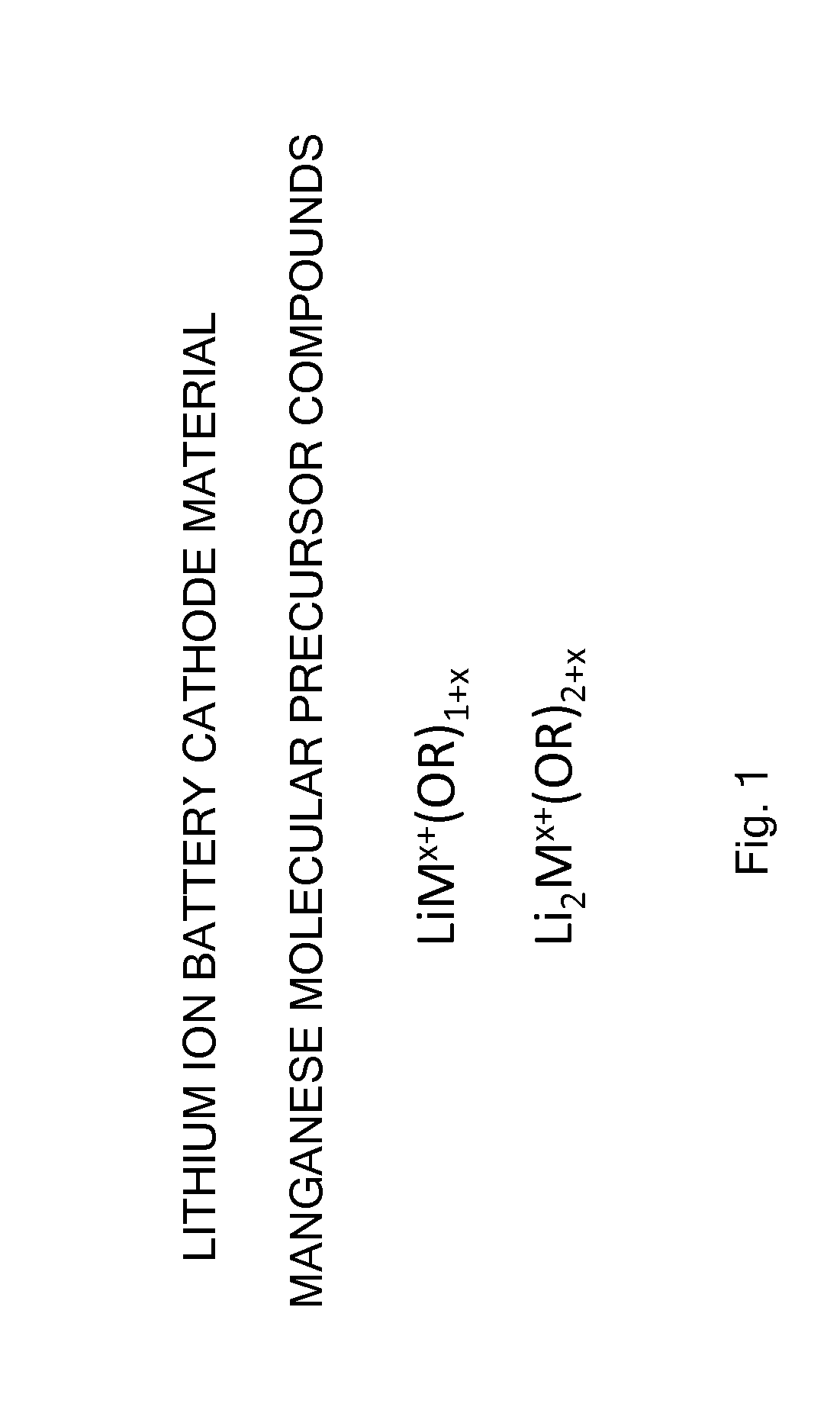
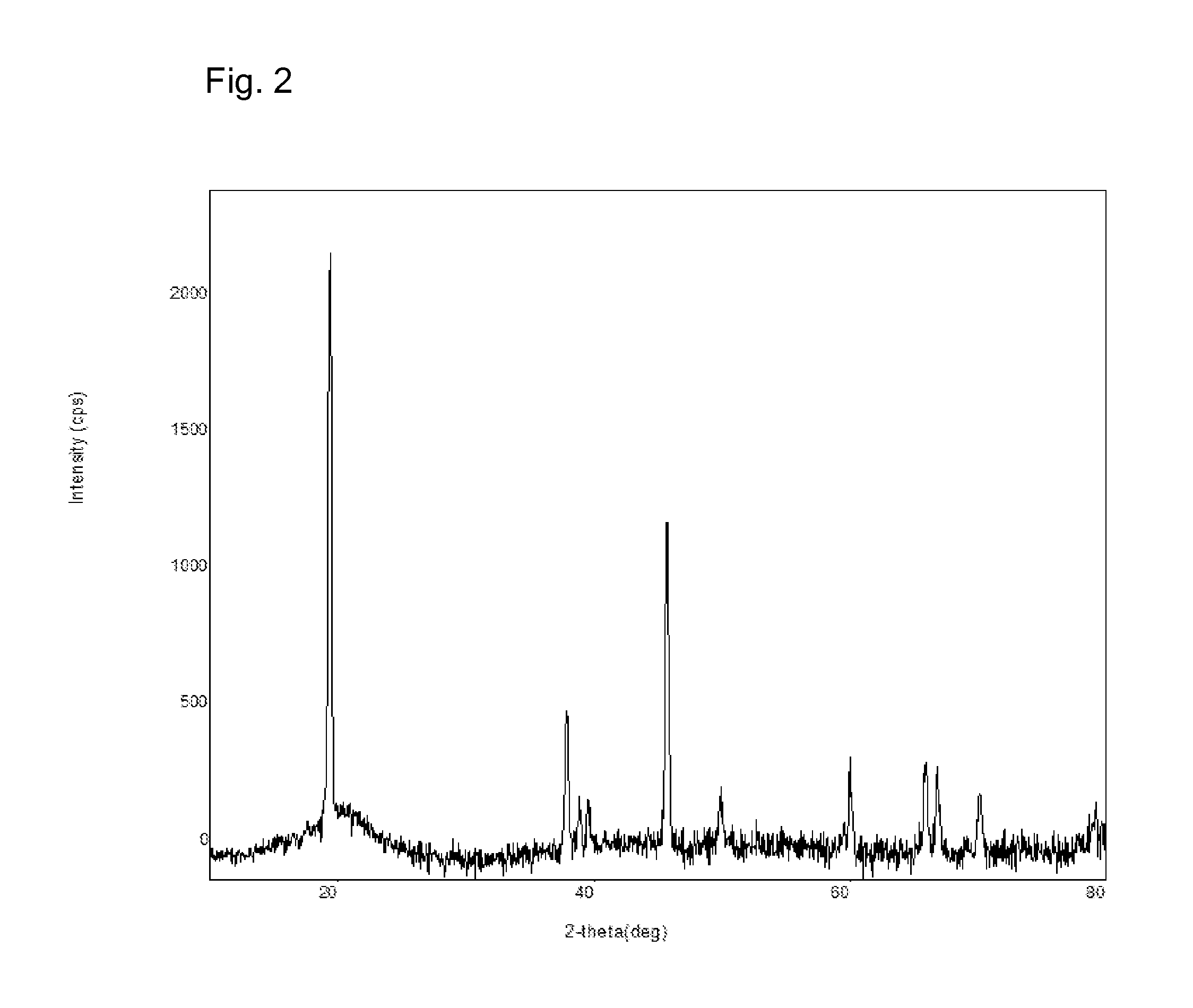
Manganese and lithium-containing molecular precursors for battery cathode materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cathode Molecular Precursor Compound LiMn(OtBu)3
[0501]A cathode molecular precursor compound represented by the formula LiMn(OtBu)3 was synthesized using the following procedure.
[0502]To a light brown solution of LiN(SiMe3)2 (0.50 g, 3.0 mmol) and Mn[N(SiMe3)2]2 (1.13 g, 3.0 mmol) in 30 mL THF was added tBuOH (0.90 mL, 9.5 mmol) using a syringe under inert atmosphere (Schlenk line). The reaction mixture remained light brown in color. The reaction mixture was stirred at 25° C. for 12 h, followed by filtration and removal of the volatile species (solvent and HN(SiMe3)2) under dynamic vacuum. 0.67 g of product (80%) was isolated as a beige solid.
example 2
Cathode Molecular Precursor Compound LiMn(OsBu)3
[0503]A cathode molecular precursor compound represented by the formula LiMn(OsBu)3 was synthesized using the following procedure.
[0504]To a light brown solution of LiN(SiMe3)2 (0.91 g, 5.4 mmol) and Mn[N(SiMe3)2]2 (2.05 g, 5.4 mmol) in 60 mL THF was added sBuOH (1.60 mL, 17.4 mmol) using a syringe under inert atmosphere (Schlenk line). The reaction mixture slowly changed color to pink / brown in 30 min. The reaction mixture was stirred at 25° C. for 12 h, followed by filtration and removal of the volatile species (solvent and HN(SiMe3)2) under dynamic vacuum. 0.92 g of product (61%) was recovered as a brown solid.
[0505]Elemental analysis by ICP: Li to Mn ratio, 1.00:1.00.
[0506]Elemental analysis by combustion (wt %): C, 50.61, H, 9.60.
example 3
Cathode Molecular Precursor Compound Li2Mn(OsBu)4
[0507]A cathode molecular precursor compound represented by the formula Li2Mn(OsBu)4 was synthesized using the following procedure.
[0508]To an orange solution of LiN(SiMe3)2 (0.84 g, 5.0 mmol) and Mn[N(SiMe3)2]2 (0.95 g, 2.5 mmol) in 100 mL THF was added sBuOH (1.2 mL, 13 mmol) via syringe under inert atmosphere (Schlenk line). The light brown reaction mixture was stirred at 25° C. for 12 h, followed by filtration and removal of volatiles under reduced pressure. 0.53 g of product (59%) was recovered as a brown solid.
[0509]The Li / Mn ratio was found to be 2.14:1.00 by use of ICP analysis.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap