Microvesicles derived from nucleated, mammalian cells and use thereof
a technology of microvesicles and mammalian cells, applied in the direction of microcapsules, chemical/physical processes, dna/rna fragmentation, etc., can solve the problems of inability to deliver drugs to specific cells or tissues, immune responses within, and inability to use red blood cell-derived vesicles to deliver drugs, etc., to reduce the agony and inconvenience of cancer patients, enhance the effect of therapeutic efficacy and easy accurate diagnosis of cells or tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Microvesicles by Extrusion
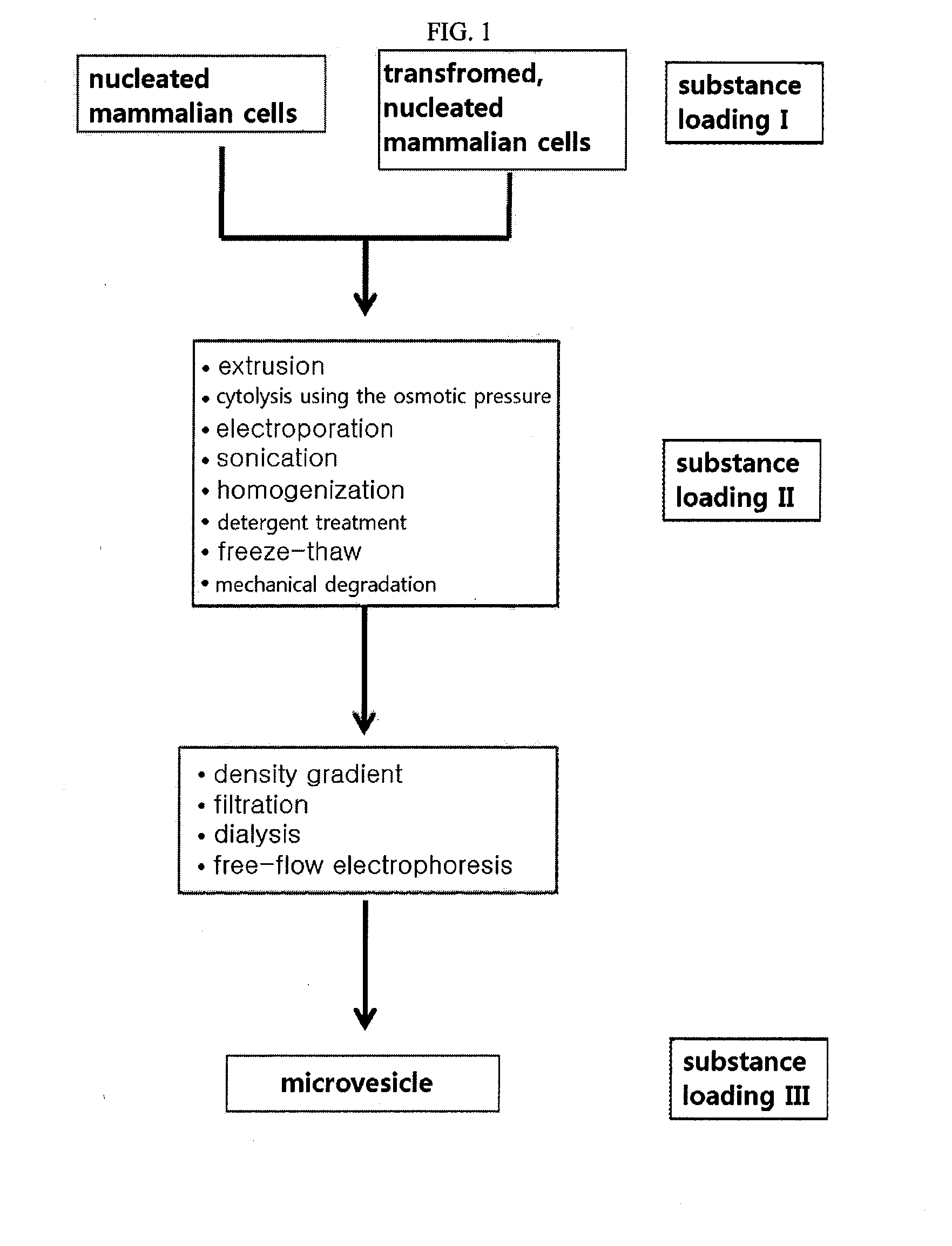
[0187]FIG. 1 is a scheme showing a process of preparing microvesicles loaded with various substances including targeting materials, therapeutic materials and diagnostic materials from nucleated mammalian cells, whether transformed or not.
[0188]According to the procedure illustrated in the scheme of FIG. 1, microvesicles were prepared from monocytes or macrophages. From among those suggested in FIG. 1, extrusion and a density gradient were selected.
[0189]The monocyte U937 (ATCC No. CRL-1593.2) or the macrophage Raw264.7 (ATCC No. TIB-71) was resuspended at a density of 5×106 cells / ml in 3 mL of PBS (phosphate buffered saline). The cell suspension was passed three times through each of the membrane filters with a pore size of 10 μm, 5 μm and 1 μm, in that order. In a 5 mL untracentrifuge tube were sequentially placed 1 mL of 50% OptiPrep, 1 mL of 5% OptiPrep and 3 mL of the cell suspension effluent from the membrane filters. Ultracentrifugation...
example 2
Preparation of Microvesicles by Sonication
[0190]According to the procedure illustrated in the scheme of FIG. 1, microvesicles were prepared from monocytes or macrophages. From among those suggested in FIG. 1, sonication and a density gradient were selected.
[0191]Monocytes or macrophages were suspended at a density of 2×107 cells / ml in 3 mL of PBS, followed by 30 cycles of sonication with the sonicator (UP 400S, Hielscher) at amplitude 50%, and cycle 0.5 and then with a water bath sonicator for 30 min. In a 5 mL untracentrifuge tube were sequentially placed 1 mL of 50% OptiPrep, 1 mL of 5% OptiPrep and 3 mL of the sonicated cell suspension. Ultracentrifugation at 100,000×g for 2 hours formed a layer of microvesicles between 50% OptiPrep and 5% OptiPrep.
example 3
Analysis of Property of Monocyte-Derived Microvesicles
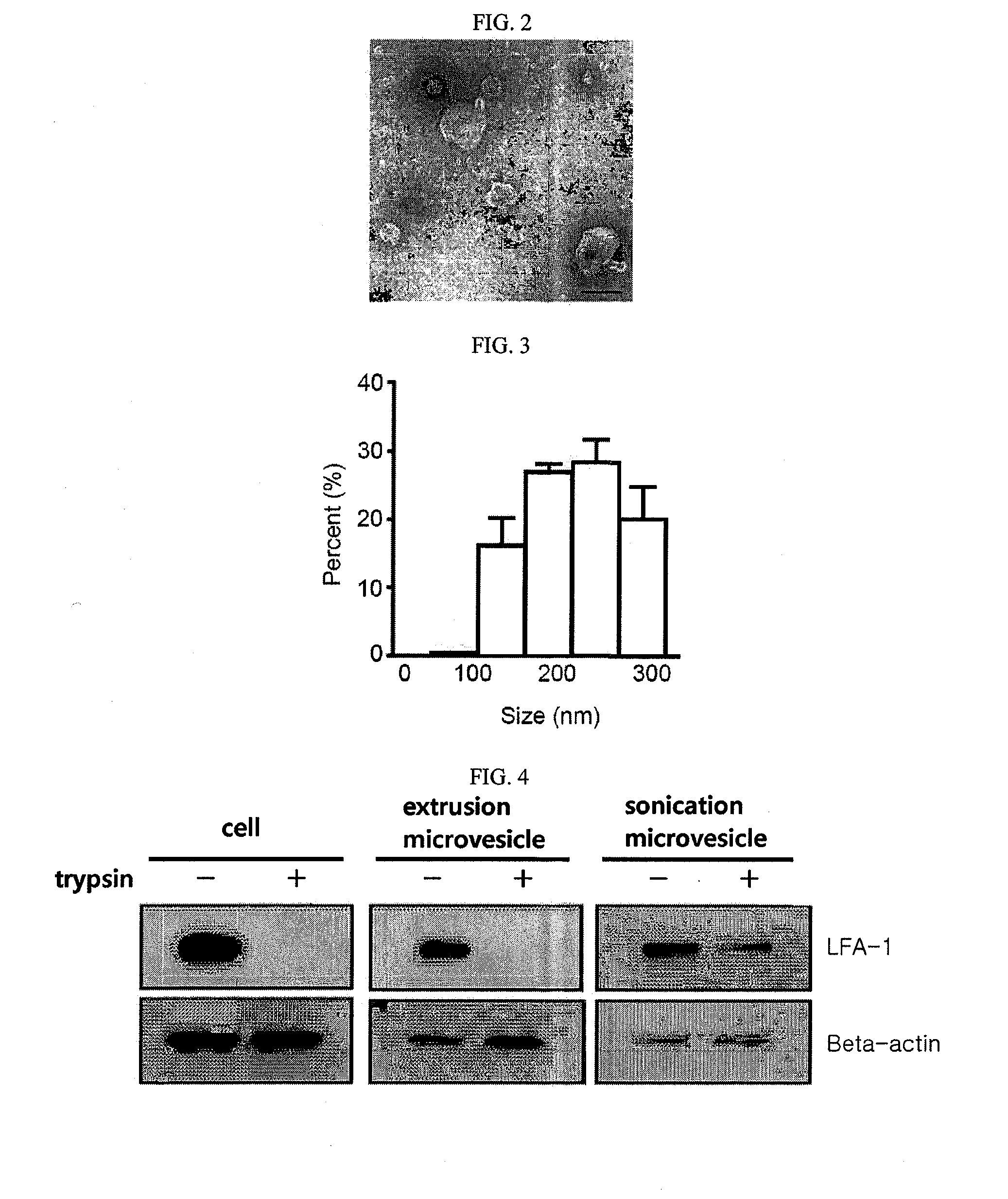
[0192]The microvesicles generated from monocytes in Example 1 were adsorbed for 3 min to a glow-discharged carbon-coated copper grid. The grid was washed with distilled water and stained for 1 min with uranylacetate before observation under a JEM101 electron microscope (Jeol, Japan). The electron microscope image is shown in FIG. 2.
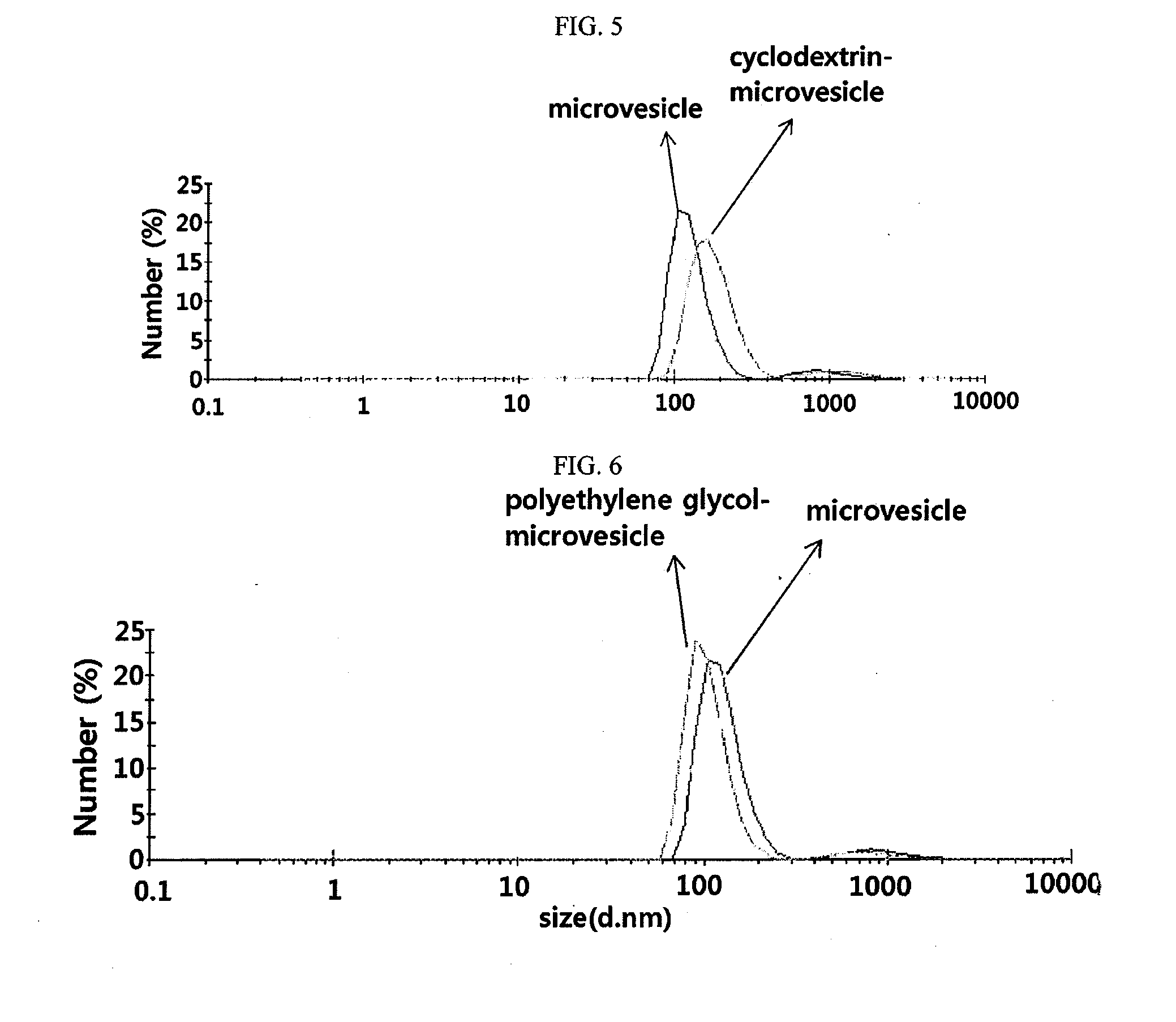
[0193]As can be seen in the Transmission electron microscope(TEM) image of FIG. 2, the microvesicles constructed from monocytes by extrusion consisted of a lipid bilayer and is generally spherical with a size of 100˜200 nm. The microvesicles generated from monocytes in Example 1 were diluted to a concentration of 5 μg / ml in 1 mL of PBS which was then placed in a cuvette and analyzed for particle sizes using a dynamic light scattering (DLS) particle size analyzer. The results are given in FIG. 3. As can be seen, the microvesicles ranged in size from 200 to 300 nm with a mean size of 250 nm.
[0194]With 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| period of time | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com