External preparation composition comprising fatty acid-based ionic liquid as active ingredient
a technology of fatty acid-based ionic liquid and composition, which is applied in the direction of biocide, drug composition, muscular disorder, etc., can solve the problems of hardly producing stable, hardly forming a uniform solution, and the system of a lower alcohol and a fatty acid ester does not always produce the sufficient effect of accelerating absorption, so as to enhance the transdermal absorbability of the drug, improve the effect of drug transdermal absorbability and excellent effect of transdermal absorb
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Eternal Preparation Composition Containing Acidic Drug (Indomethacin)
(1) Composition of Indomethacin and Salt Thereof and its Transdermal Absorbability
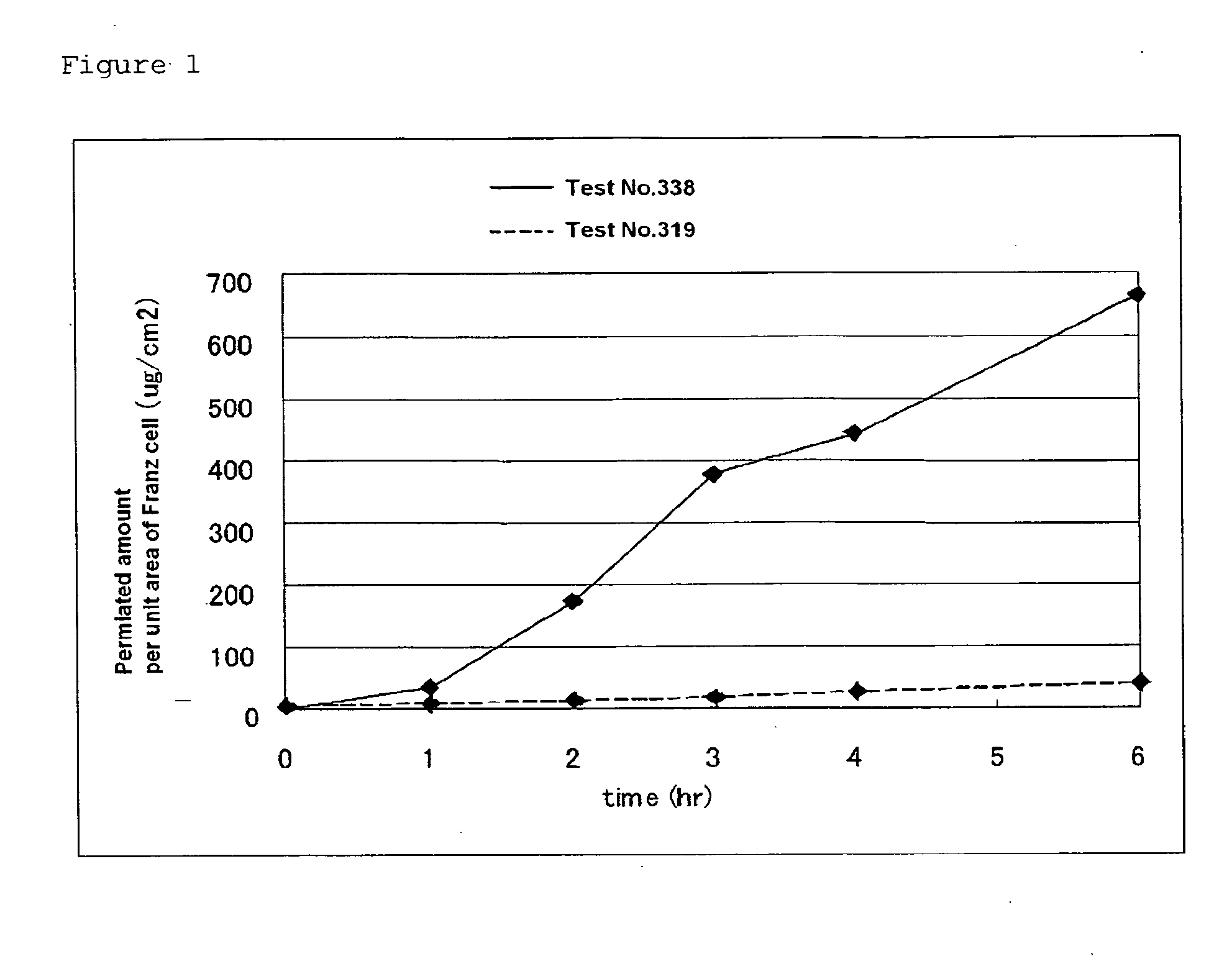
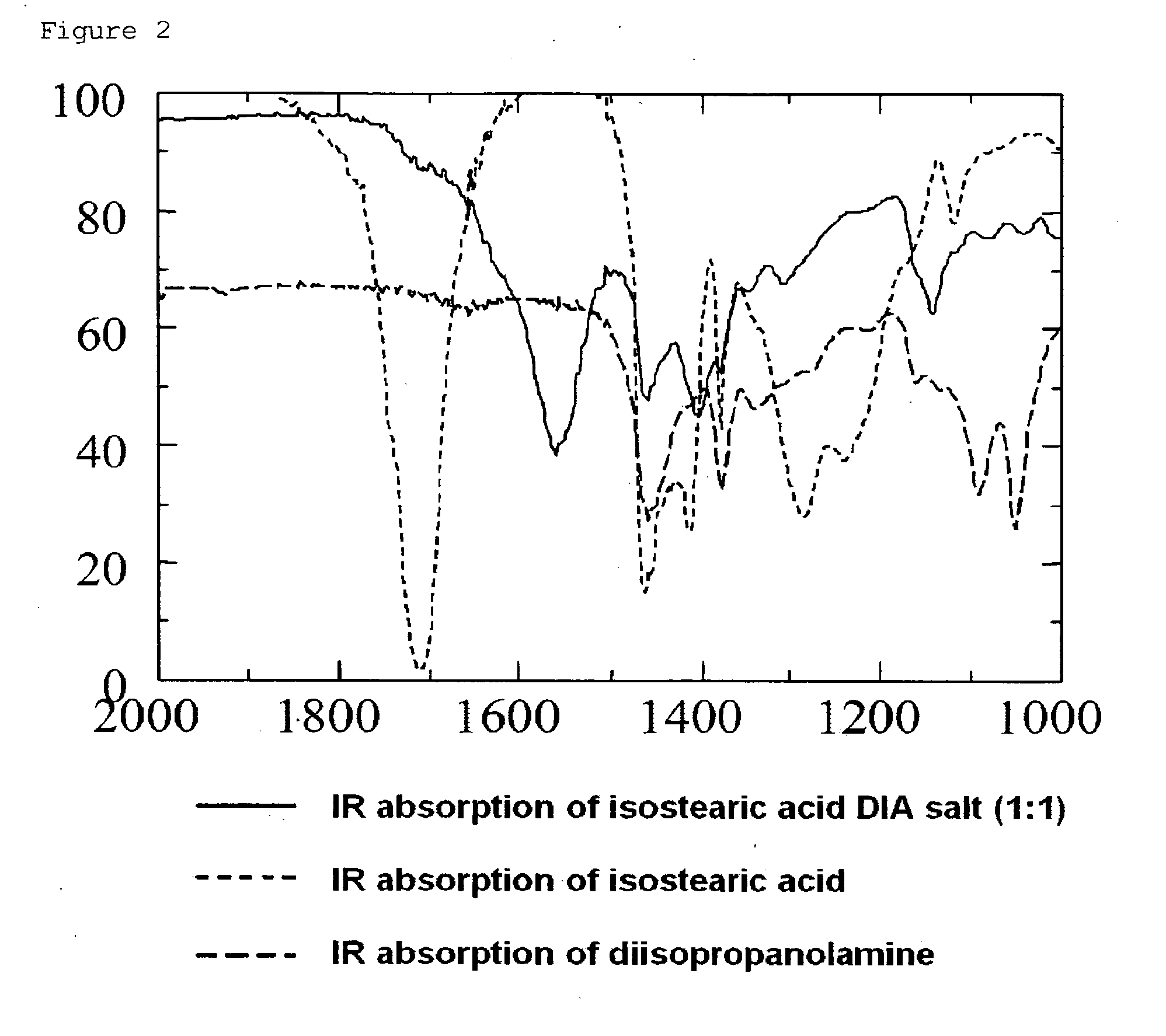
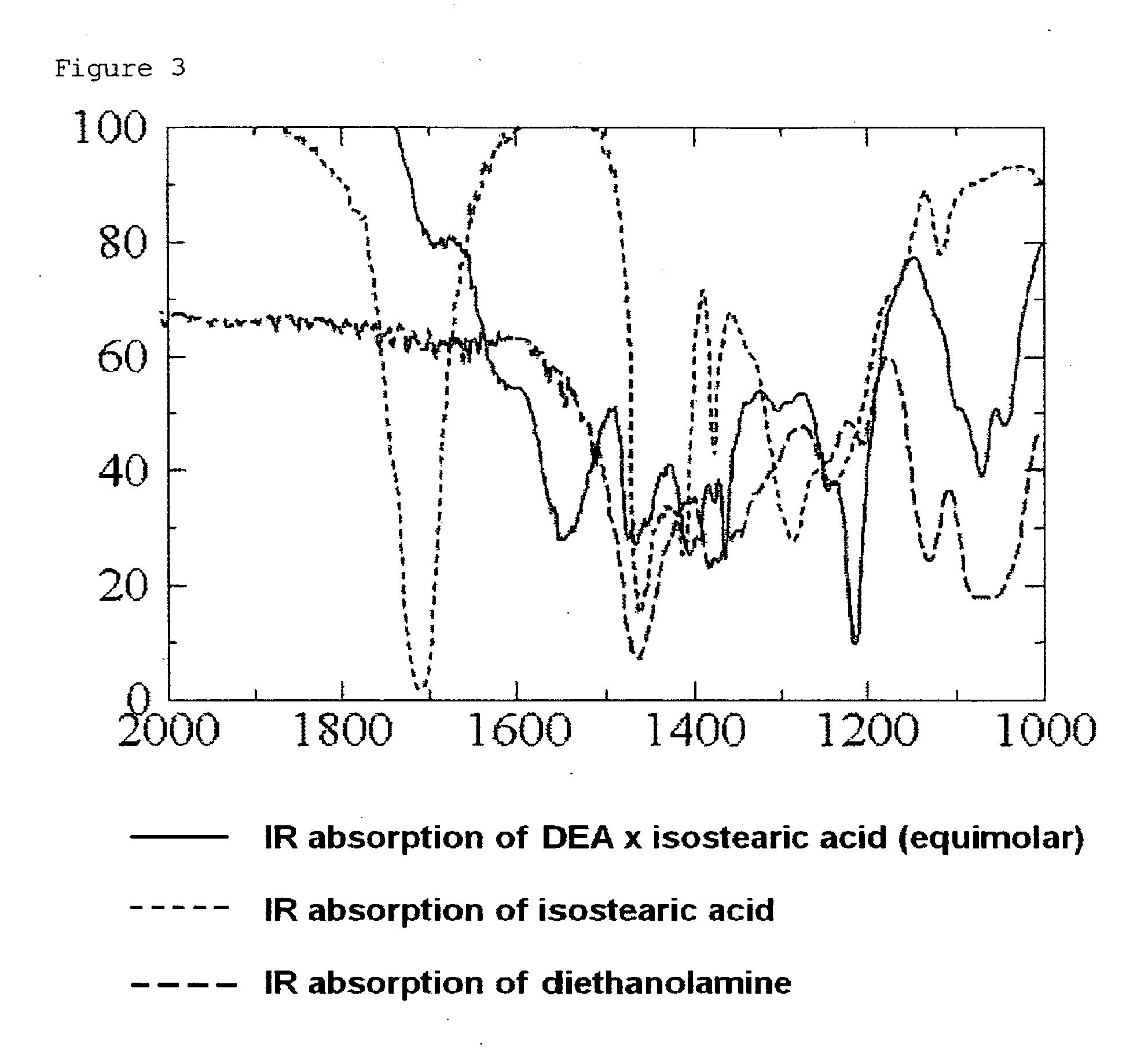
[0085]100 mg (0.28 mM) of indomethacin is weighed, and in case of preparing a salt thereof, an equimolar amount of an organic base or the like is added thereto. Furthermore, isostearic acid / diisopropanolamine (SDE / PG) was added thereto as a fatty acid-based ionic liquid to adjust the whole amount to 1 g. This sample is directly used. Alternatively, the sample was diluted 4-fold with a solvent to prepare 4 ml of a solution (indomethacin concentration: 2.5%). 100 μl of the solvent-diluted solution was weighed and subjected to a skin permeation test using Franz-cells according to Test Example 1. As shown in Table 1 below, a salt of indomethacin was prepared using an organic base and a basic drug.
[0086]In this way, an external preparation composition containing indomethacin or an equimolar salt of indomethacin dissolved in the fatty acid-...
example 2
Transdermally Absorbable Composition Containing Acidic Drug (Flurbiprofen)
[0119]According to Example 1(1), an external preparation composition was prepared according to the composition (w / w %) of Table 10 below using flurbiprofen. The composition was measured and evaluated for its transdermal absorbability according to Test Example 1 using Franz-cells. The transdermal absorbability was evaluated based on a cumulative amount permeated 6 hours after the start of the test.
[0120]The results are also shown in Table 10 below.
TABLE 10Evaluated drugTest No.(Flurbiprofen content)#1#2SolventReferenceFlurbiprofen 2.5%80SDEExample (23)97.5151Flurbiprofen 2.5%64022.5SDE / PG37.5 / 37.5ReferenceFlurbiprofen lidocaine80100Example (24)equimolar salt 2.5%118Flurbiprofen lidocaine66820.1SDE / PGequimolar salt 2.5%37.5 / 37.5122Flurbiprofen lidocaine60020.1MIP / PGequimolar salt 2.5%37.5 / 37.5[Note]#1 Transdermal absorbability (μg / cm2)#2 Fatty acid-based ionic liquid isostearic acid / DIAThe abbreviations DIA, SDE...
example 3
Transdermally Absorbable Composition Containing Acidic Drug (Etodolac)
[0124]According to Example 1(1), an external preparation composition was prepared according to the composition (w / w %) of Table 11 below using etodolac. The composition was measured and evaluated for its transdermal absorbability according to Test Example 1 using Franz-cells. The transdermal absorbability was evaluated based on a cumulative amount permeated 6 hours after the start of the test.
[0125]The results are also shown in Table 11 below.
TABLE 11Evaluated drugSolventTest No.(Etodolac content)#1#2SDE / PGReferenceEtodolac 16050 / 50Example (40)2.5% (#3)(#4)139Etodolac40922.537.5 / 37.52.5%140Etodolac lidocaine43720.537.5 / 37.5Equimolar salt 2.5%[Note]#1 Transdermal absorbability (μg / cm2)#2 Ionic liquid isostearic acid / DIA(#3) data from mixed system of 5 types of NSAIDs (ketoprofen, flurbiprofen, etodolac, indomethacin, and loxoprofen).(#4) Separated into two layers The abbreviations DIA, SDE, and PG are as defined ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com