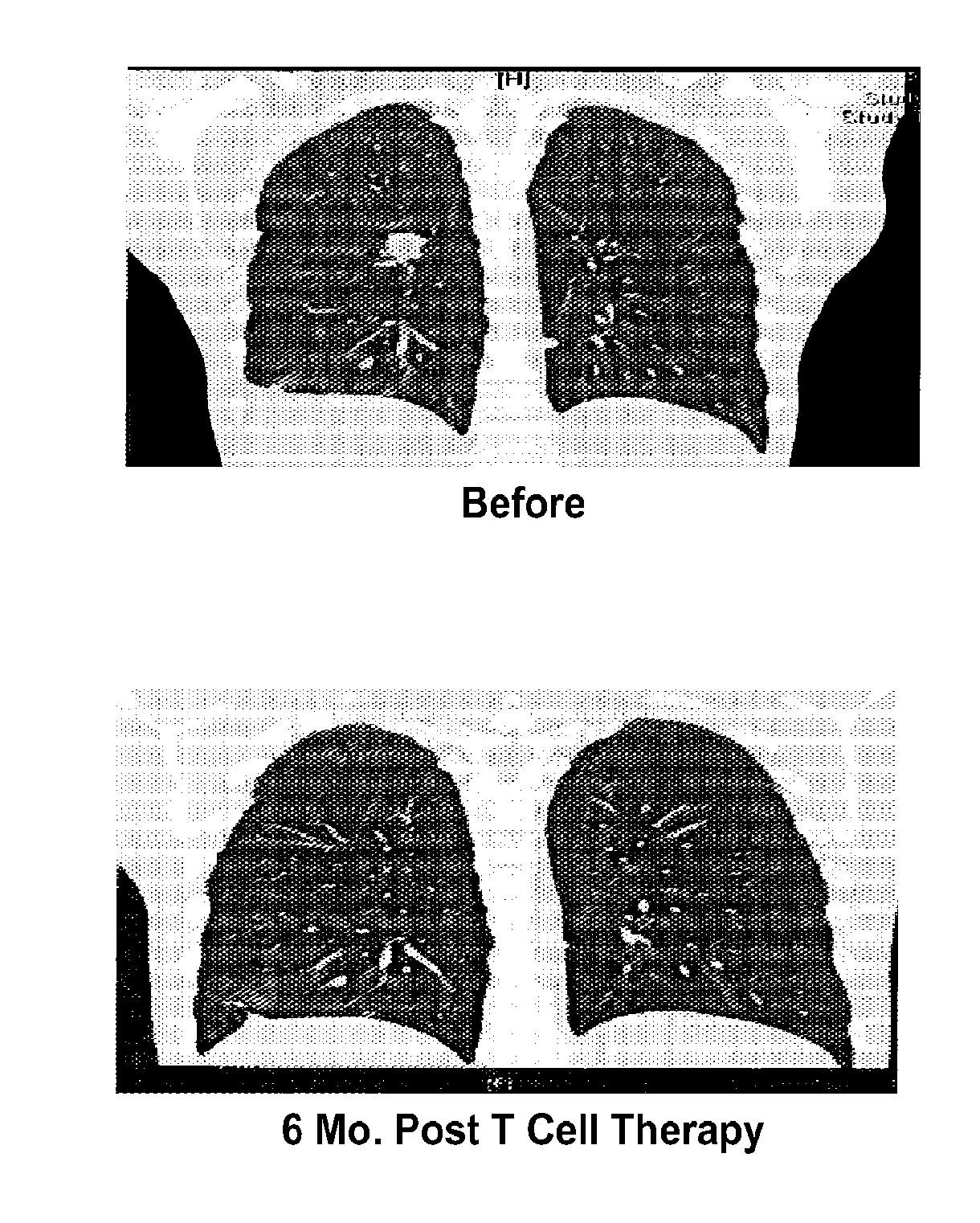
Modulated Immunodominance Therapy
a technology of immunodominance and immunomodulation, applied in the field of cancer, chronic infections, autoimmune diseases, transplantation, etc., can solve the problems of unfavorable primary immunosuppressive environment and inability to create a profile of immune response on a routine clinical basis, so as to alter the immunodominance hierarchy of patients and alter the immunodominance hierarchy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1. Example 1
EBV Latent Infection, Lymphomas and Nasopharyngial Carcinoma
[0161]90% of the world's population has been exposed to EBV (the causative virus in mononucleosis) as measured by antibodies in the blood. EBV becomes latent in B cells and shuts off the majority of its proteins but express very low levels of latent antigens LMP1, LMP2 and sometimes EBNA-1. These proteins are weakly immunogenic but are required to maintain the virus even in its latent state. Because they derive predominantly from B cells, 40% of lymphomas test positive for EBV latent antigens. Thus, these antigens can serve as targets for the generation of CTL responses in adoptive cell therapy. In addition, Nasopharyngial carcinoma also expresses EBV latent antigens as do other tumors (e.g. −10% of gastric cancer). While CTL's have been used to treat EBV lymphomas, the current production methods are time consuming (3-6 months) and cumbersome using B cells transformed with EBV as repeat stimulation. Further, CTL...
experiment 1
a. Experiment 1
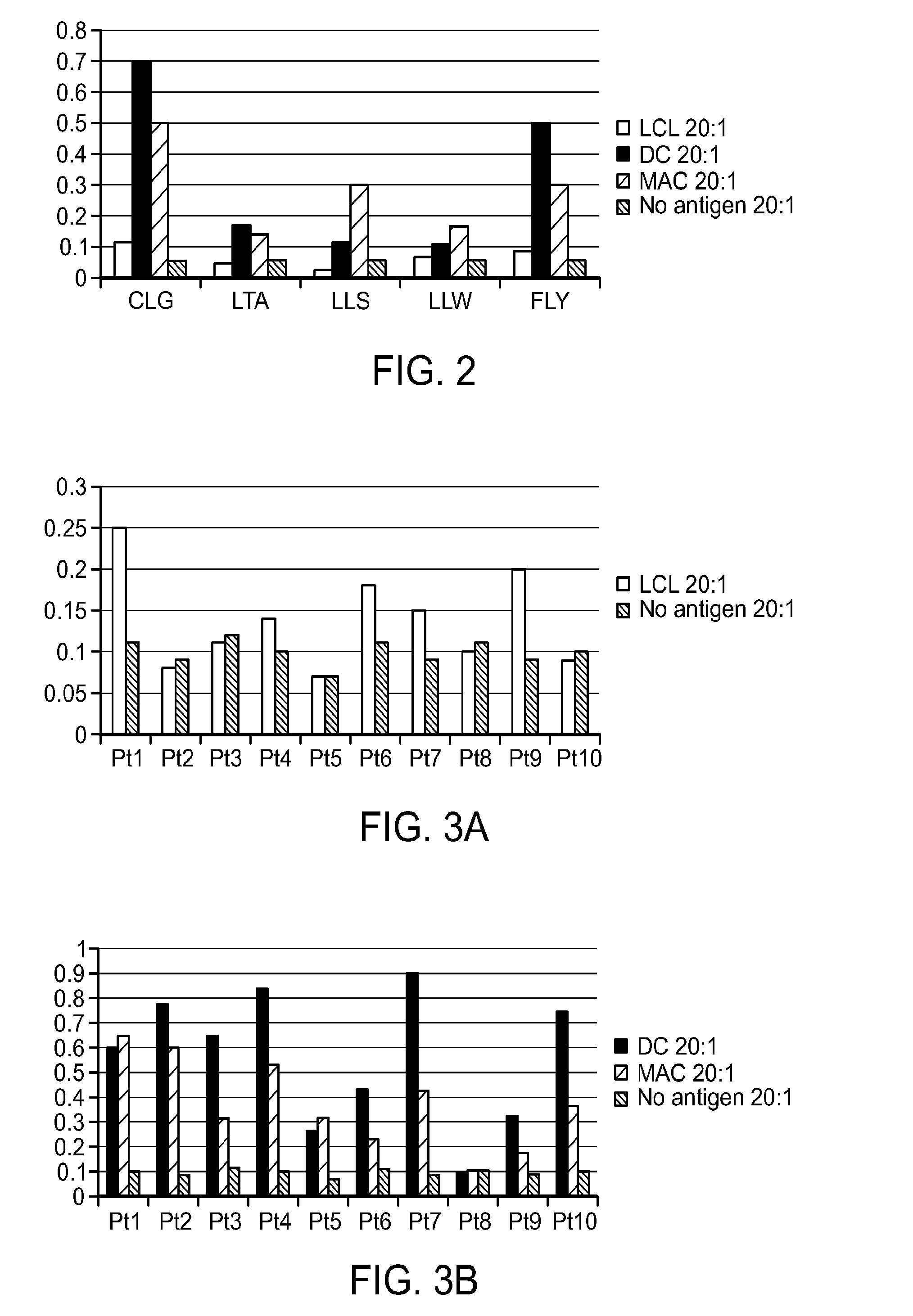
The Relative Frequency of T Cells Responding to Subdominant Epitopes is Enhanced when Dendritic Cells or Activated Macrophages are Used as Antigen Presenting Cells Instead of LCL
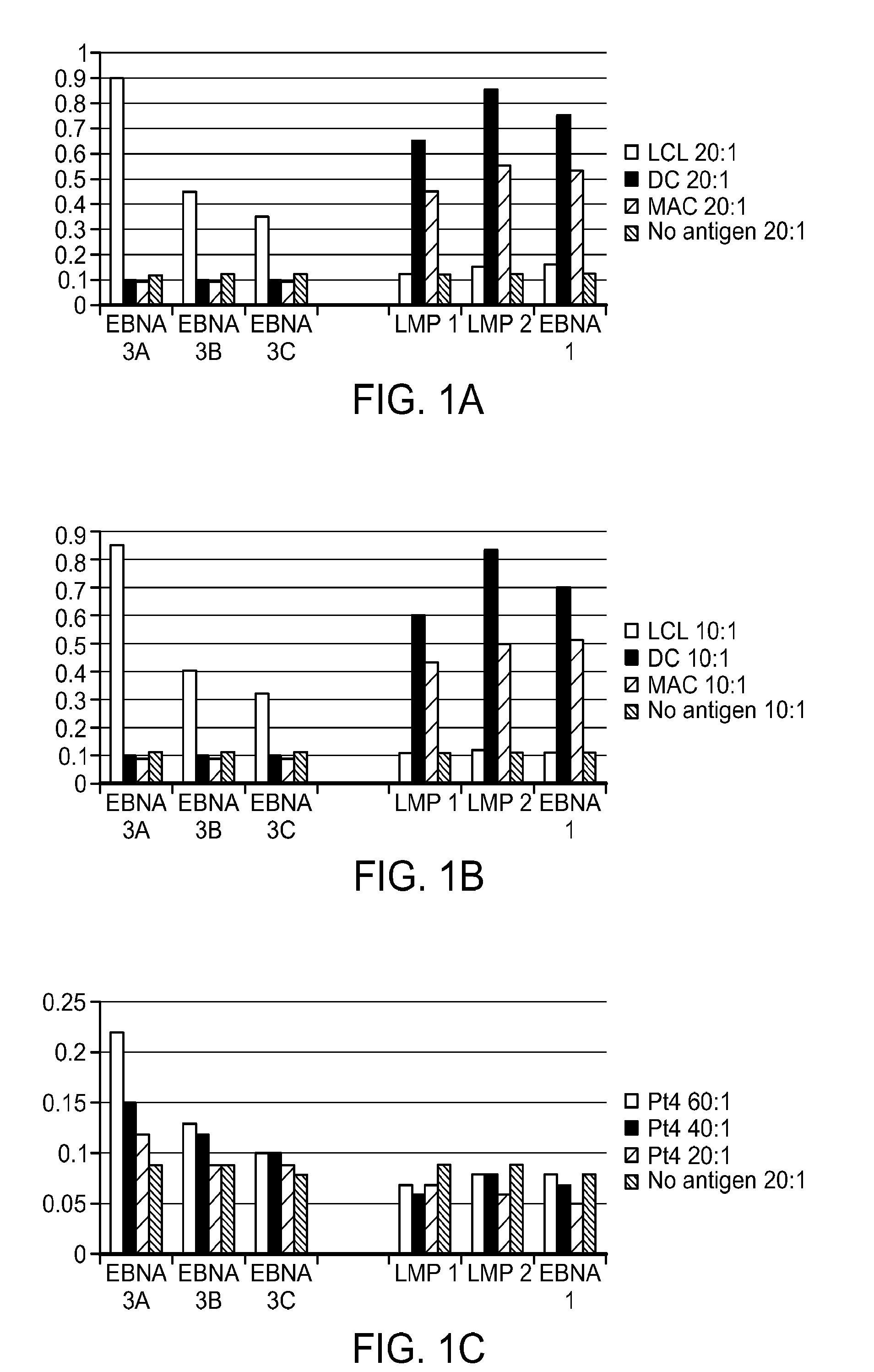
[0166]T cell lines were prepared from 10 patients using 3 different antigen presenting cells as stimulators: EBV transformed lymphoblastoid cell lines (LCL) presentation and expansion; Dendritic cell (DC) presentation with cytokine expansion; IFNγ Macrophage (MAC) presentation with cytokine expansion. Each of the 3 were stimulated during antigen presentation with a mixture of 3 plasmids expressing subdominant epitopes of EBNA-1 (EBNA-1 with deletion of aa 90 to 325), LMP1 (LMP1 with deletion of aa 1-43 and aa 260-315) and LMP2 (LMP2A in 2 plasmids one expressing aa 1-399 and the second plasmid expressing aa 400-497). The T cell lines so generated using the protocol of the invention were then studied in 51Cr release assay.
[0167]FIGS. 1A and 1B: 51Cr release at two different effector:target (E / T...
example 2
b. Example 2
[0191]In the following experiments, T cells were grown in the same way as outlined in Example 1 above, with different variations designed to enhance antigen processing to favor T cells reactive with the production of subdominant epitopes.
[0192]FIG. 7: (51Cr release) Cr release at 20:1 effector:target (E / T) ratios (CTL Lines: HLA matched fibroblasts) HLA matched fibroblasts pre-pulsed with peptides representing Dominant antigen (EBNA-3A), Subdominant antigen (LMP2) and specific HLA A2 restricted subdominant epitopes from LMP2 with CTLs made from the same patient using two different methods: one with 100 nM to 300 nM bortezomib added during antigen presentation, one without.
[0193]Conclusion: Addition of the Proteosome antagonist bortezomib during CTL antigen presentation modifies antigen processing to generate CTL's to a broader number of subdominant epitopes with a modest decrease in the response to dominant epitopes
[0194]FIG. 8: (51Cr release) Cr release at 20:1 effector...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Responsivity | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com