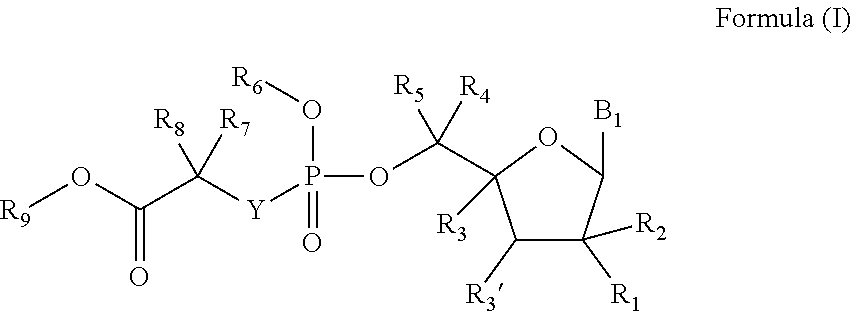
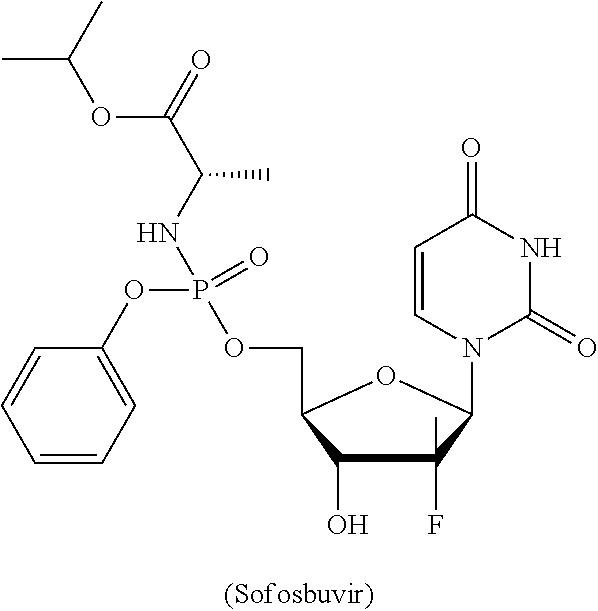
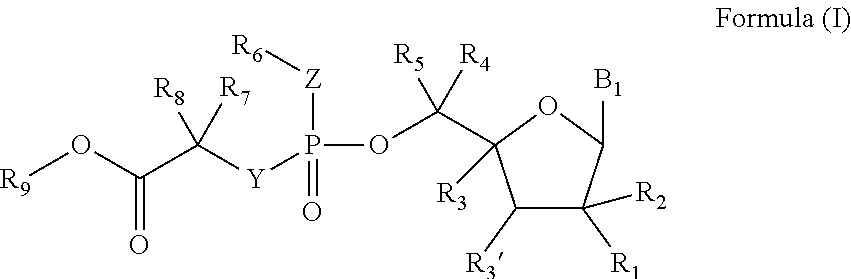
Deuterated nucleoside prodrugs useful for treating hcv
a nucleoside prodrug and hcv technology, applied in the direction of drug compositions, group 5/15 element organic compounds, peptide/protein ingredients, etc., can solve the problems of patient exhibiting serious adverse events such as vomiting/nausea, and reducing the efficacy, and achieve the effect of effective treatment against hepatitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of (S)-Isopropyl 2-(((S)-(((2R,3R,4R,5R)-5-(5-Deutero-2,4-Dioxo-3,4-Dihydropyrimidin-1(2H)-yl)-3,4-Dihydroxy-4-Methyltetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate (Prodrug of 2′-Methyl-5-Deutero-Uridine)
[0268]
[0269]Tetrabenzoylsugar (1, 2.44 g) and 5-Deutero-Uracil (2, 1.0 g) were reacted following the literature procedure described in Harry-Okuru et al. (J. Org. Chem. (1997) 62: 1754), followed by debenzylation using NaOMe / MeOH to give 2′-Methyl-S-D-Uridine (3, 0.8 g). Compound 3 (0.7 g) was converted to its phosphoramidate derivative 5 (0.63 g) following literature procedure described by Ross et al.
example 2
Preparation of (S)-Isopropyl 2-(((R)-(((2S,3R,4R,5R)-5-(2,4-Dioxo-3,4-Dihydropyrimidin-1(2H)-yl)-3,4-Dihydroxy-4-Methyltetrahydrofuran-2-yl)dideuteromethoxy)(phenoxy)phosphoryl)amino)propanoate (Prodrug of 2′-Methyl-5′,5′-DiDeutero-Uridine)
[0270]
[0271]Compound 6 was prepared from 2′-Me-Uridine (A) following procedure reported by Corey et al (J. Org. Chem. (1984) 49: 47350 with some modifications.
[0272]Lithium chloride (1.76 g) was stirred with NaBD4 (1.58 g) in EtOD for 1 h. Compound 6 (2.97 g) was added to this solution and stirred at room temperature for 3 h and quenched with acetic acid-d, diluted with ethyl acetate, washed with brine and evaporated to dryness. The residue was purified by chromatography over silica gel to give 5′-dideuterated compound 7 (2.1 g). Compound 7 (2.1 g) was treated with trifluoroacetic acid in presence of water to give the dideuterated nucleoside 8 (1.52 g). Compound 8 (1.0 g) was converted to its phosphoramidate derivative 9 (0.78 g) following literat...
example 3
Preparation of (2S)-Isopropyl 2-(((((2S,3R,4R,5R)-1-(6-Deutero-5-(trideuteromethyl)pyrimidine-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)3,4-dihydroxy-4-methyltetrahydrofuran-2-yl)dideuteromethoxy)(phenoxy)phosphoryl)amino)propanoate (Compound 16)
[0273]
[0274]Tetrabenzoyl sugar 1 and tetradeutero thymine 10 (2.1 equivalents; prepared according to the procedure in Heterocycles (2005) 66:361) were reacted following the literature procedure described in Harry-Okuru et al. (J. Org. Chem. (1997) 62: 1754), followed by debenzylation using NaOMe / MeOH to give compound 11.
[0275]Excess 2, 2-Dimethyl propane is added to compound 11 in acetone. The resulting mixture was cooled in an ice bath for 30 min, then p-Toluenesulfonic acid (1.3 equivalents) was added and the reaction mixture was stirred at room temperature for 24 hrs. After completion of the reaction (monitored by HPLC), the reaction mixture is cooled in an ice bath for 30 minutes and neutralized using cold aqueous potassium carbonate. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com