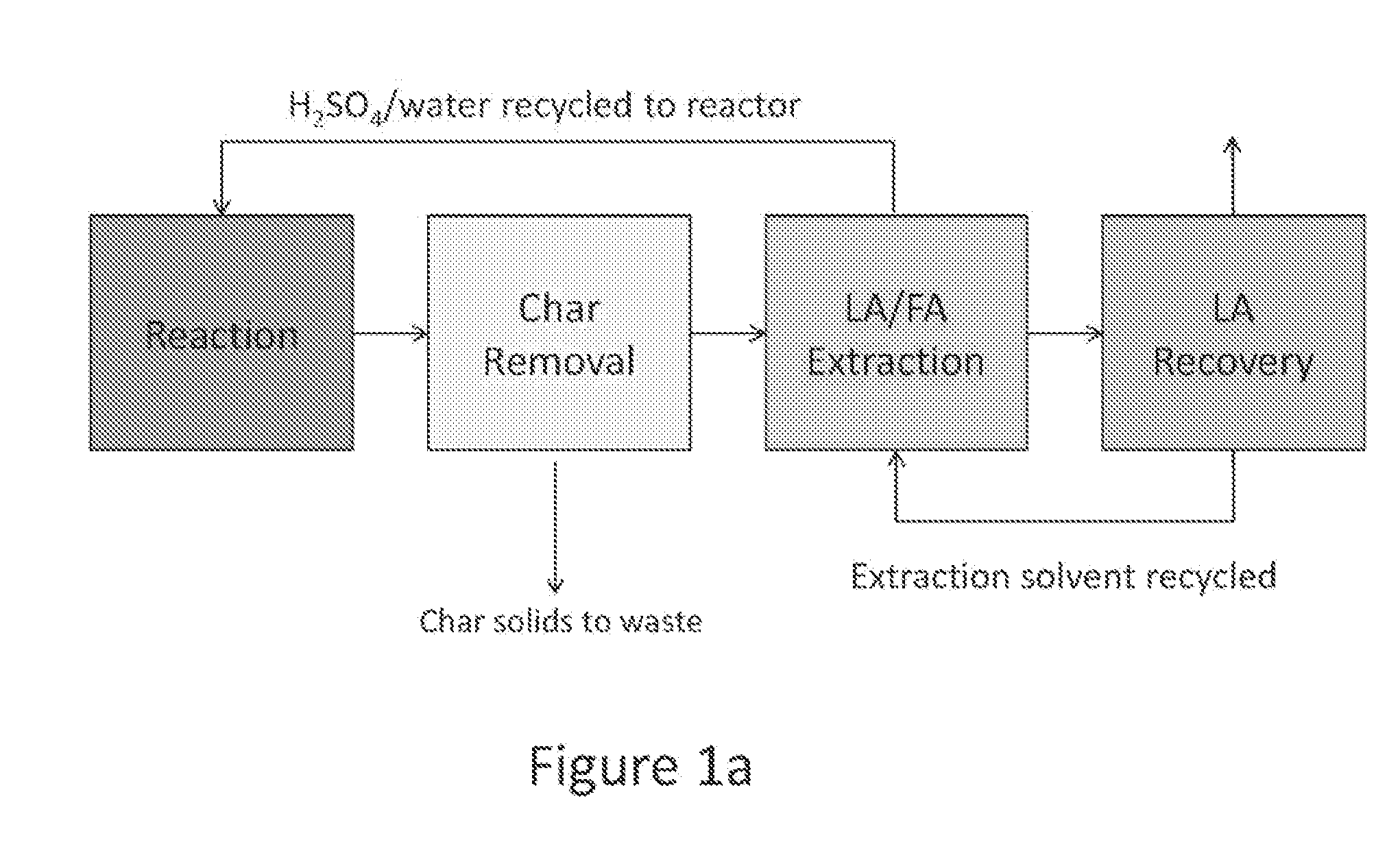
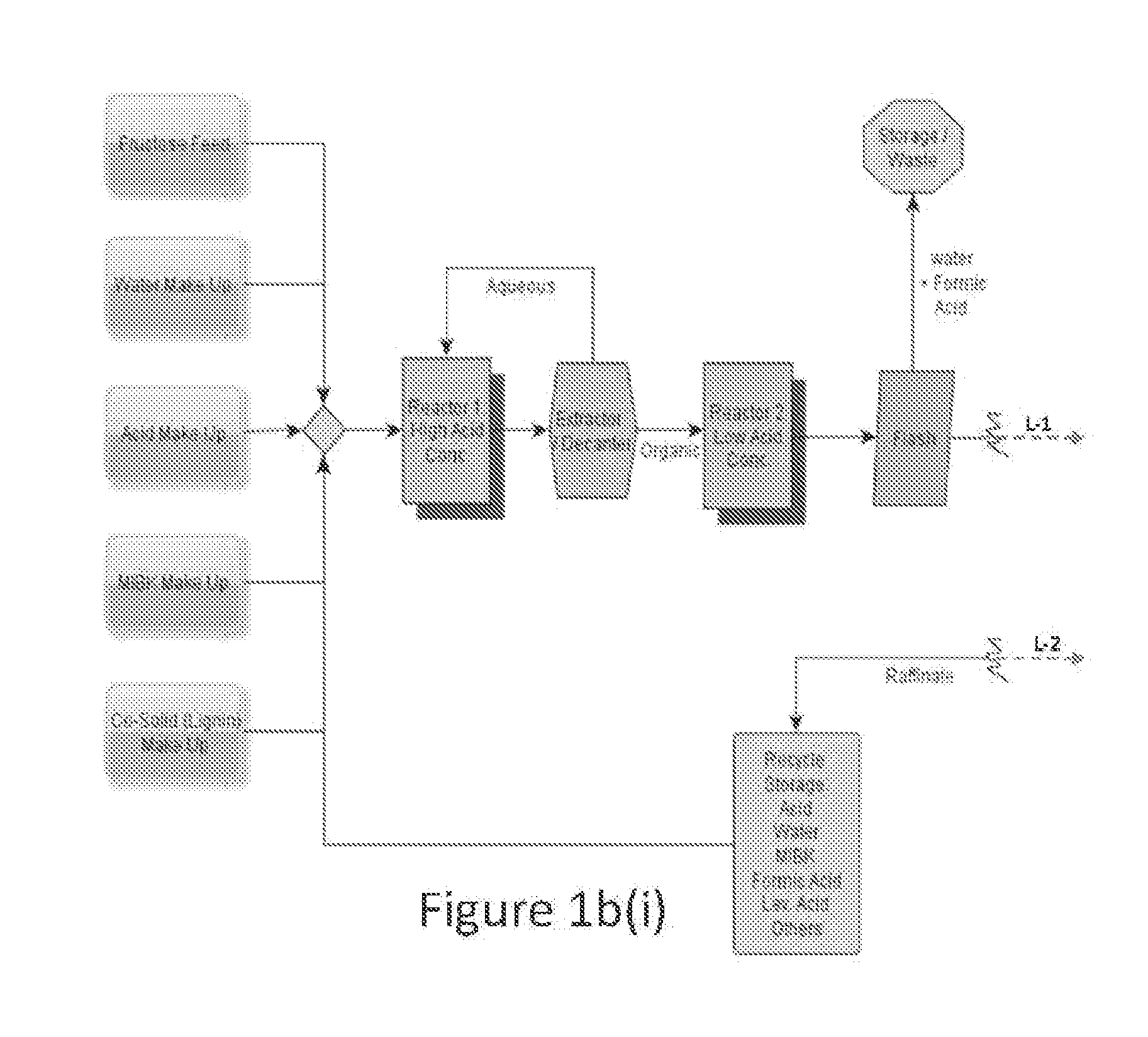
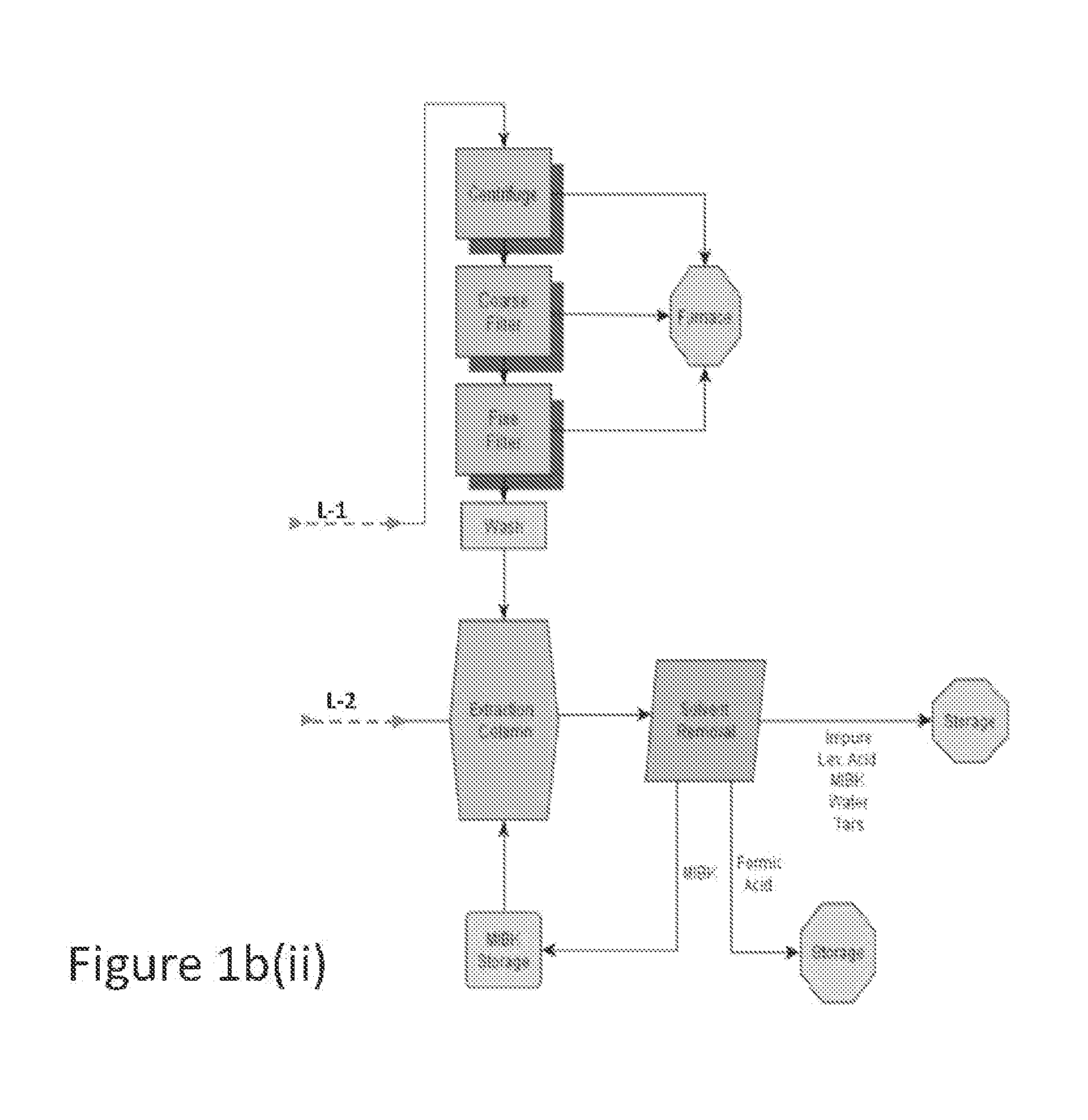
Process to prepare levulinic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0494]Into a 1 L hastelloy parr reactor 135.12 g fructose (94% purity, 0.75 mol), 500 mL DI water, and 38.17 g sulfuric acid were charged. The reactor was sealed and the reaction mixture was heated up to a temperature of 150° C. while stirring at 52 RPM. Once the reaction mixture reached a temperature of 150° C., the mixture was held at that temperature for 1 hour. After the 1 hour reaction time the heat was turned off and the heating mantle was lowered and the reactor was cooled using an ice water bath. Once the reactor was cooled it was dismantled and the reaction mixture was filtered through a 0.45 μm filter using vacuum filtration in order to remove the char from the liquid. The liquid was analyzed by HPLC and found to contain 9.9 wt % levulinic acid. The liquid is referred to as “hydrolysate”.
[0495]Into a 1 L separatory funnel 300.10 g hydrolysate and 300.07 g Methyl isobutyl ketone (MIBK) were charged. The separatory funnel was shaken in order to mix everything together and th...
example 2
[0503]1 Mol / L D-Fructose (15 mL) was prepared by diluting 2.44 g of crystalline D-Fructose (93.5% purity, 6.5% moisture, Aldrich) up to 15.0 mL with DI water. The 15.0 mL was transferred to a 3 oz. empty high pressure, high temperature reaction vessel, and concentrated sulfuric acid (407 μL) was added. The reaction vessel was capped using a Teflon sleeve, an o-ring, rubber washer and a stainless steel plug. The reactor was securely closed with stainless steel couplings. The reaction vessel was placed into a 180° C. hot oil bath to reach an internal temperature of around 160° C. After a specified reaction time, the reaction vessel was then removed from the hot oil and placed in a room temperature water bath for 1 minute to begin cooling. Following the room temperature water bath, the reactor was placed in an ice water bath to quench the reaction. Once the reactor vessel had cooled, it was opened, and the contents were filtered, weighed, and then analyzed by HPLC. The humin solids tha...
examples 3-4
[0504]The procedure outlined in Example 2 was repeated, except that the feed concentration of fructose and acid catalyst was varied, as well as, the temperature of the reaction.
TABLE IWt % LAYieldImprovementWt %MolarMolarafterWt %SulfuricFructoseYieldYieldWashingFructoseacid inMax.TimeConversionof LAof FASolidExamplein FeedfeedTemperature(min)(%)(%)(%)Humins213.64.7143° C.50100546616318.95.5156° C.301006376.912423.55.5160.1° C. 30100627915
[0505]As can be seen from Table I, fructose can be hydrolyzed completely in less than 60 minutes of reaction time to afford up to 63 mol % yield and 79 mol % yield of formic acid (FA). Also, extracting LA from the solid humin material resulted in >10 wt % yield improvement of LA in all of the examples.
[0506]Utilizing High Fructose Corn Syrup, inulin, oligomeric fructan polymers, and the like are also be useful in this invention.
[0507]Another process of this invention involves the pretreatment of glucose to obtain >70% conversion to fructose direct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com