System and method for the production of recombinant glycosylated proteins in a prokaryotic host
a technology of glycosylated proteins and prokaryotic host, which is applied in the field of expression systems, can solve the problems of difficult cell line culture, difficult to keep sterile, and inability of presently used biotechnological processes to generate glycoproteins, etc., and achieves the effect of convenient handling and growing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
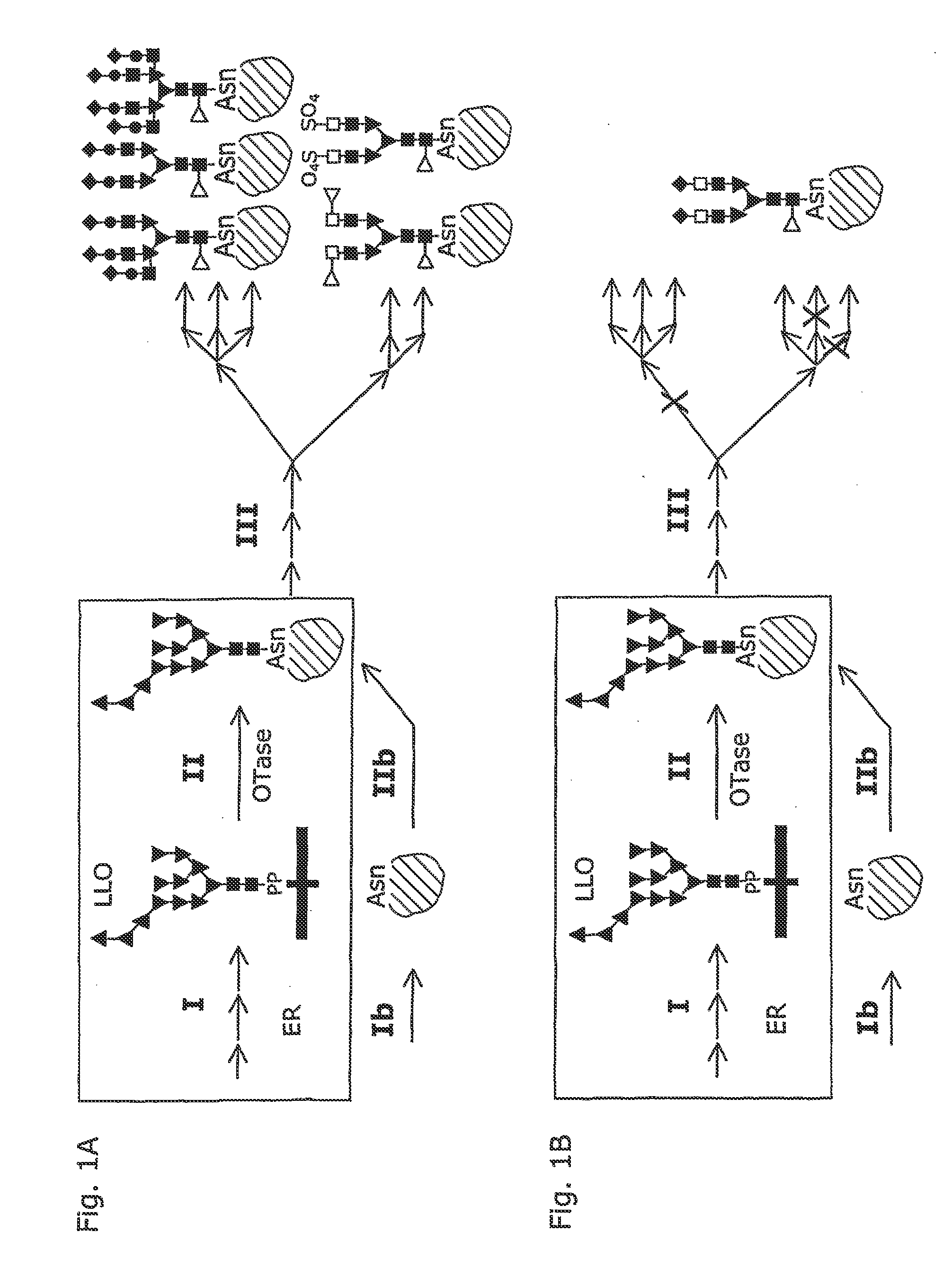
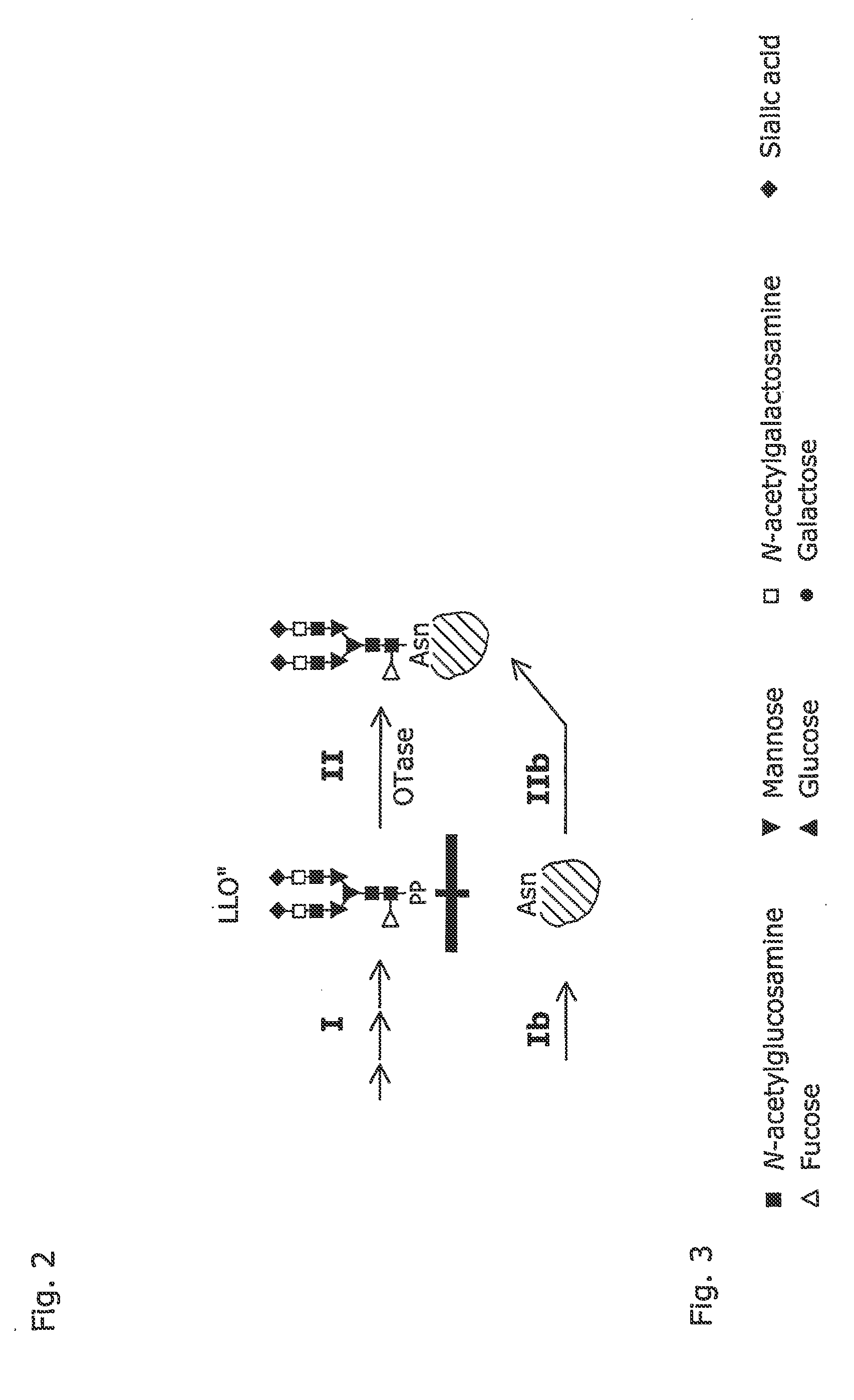
[0039]The present invention bases on the finding, that Campylobacter jejuni, a gramnegative bacterium, produces glycoproteins. Utilizing methods known per se, we have introduced the C. jejuni gene encoding AcrA, a glycoprotein, into E. coli. This results in the expression of non-glycosylated AcrA protein (see FIG. 2, step Ib). Subsequently and again utilizing known methods, an operon of C. jejuni en coding a) specific glycosyltransferases and b) an OTase was introduced into E. coli. This resulted in the production of specifically glycosylated AcrA protein according to the invention (see FIG. 2, steps I and II), as verified—always using methods known to skilled persons—by the binding of a highly specific lectin and glycosylation specific antibodies to the heterologously produced AcrA protein [Michael Wacker et al. (2002) N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli (SCIENCE, Vol 298: 1790-1793]. In addition, the structure of the oligosacchar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| structure | aaaaa | aaaaa |
| physical properties | aaaaa | aaaaa |
| thermal stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com