Anthocyanin-based colorant
a technology of anthocyanin and colorant, applied in the field of natural colorants, can solve the problems of unsuitable for use in food coloring applications, unsuitable for vegetarian consumers, red radish, etc., and achieve the effect of improving colorant properties, reducing the overall flavor and taste of the colorant, and being easy to achiev
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Characterization of Major Anthocyanins From RSWP
A) Extraction
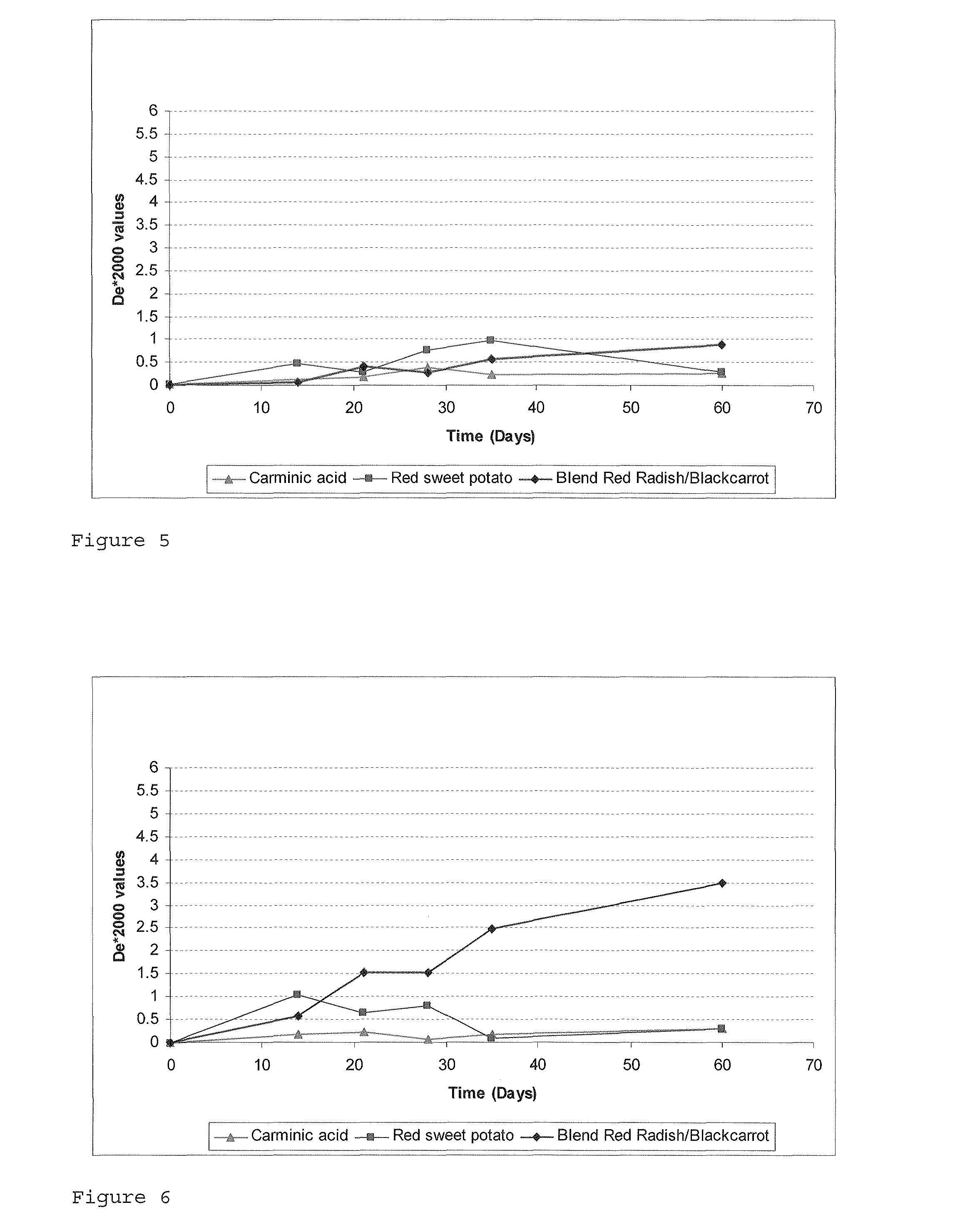
[0077]A RSWP concentrate prepared by chopping RSWP tubers into slices and “washing” them 4 times with acidified water to conduct an extraction, and then subjecting the obtained extract to micro-filtration and subsequent purified on an absorbing resin. The resulting anthocyanin extract was concentrated and then pasteurized.
[0078]Anthocyanins were isolated and concentrated on a Sep-Pak C18 cartridge (Waters®). The cartridge was washed with 2 ml of methanol and then with 2 ml of acidified water (HCl 0.3%) before loading few drops of RSWP concentrate. Once the cartridge has been washed with 4 ml of acidified water (HCl 0.3%), and then with 2 ml of ethyl acetate, anthocyanins were finally eluted with a minimum volume of acidified methanol (HCl 0.1%).
B) Analysis
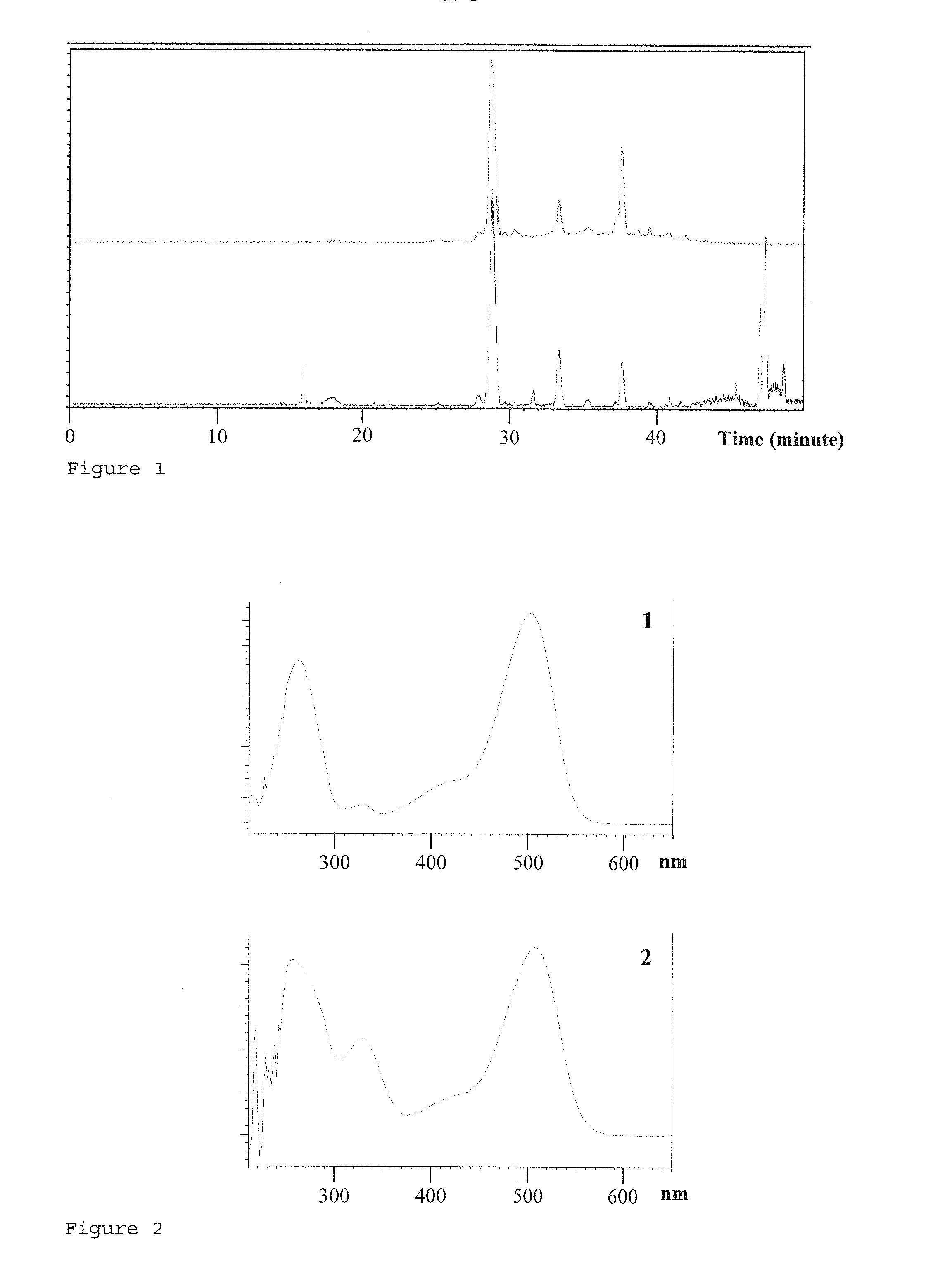
[0079]The anthocyanin extract was analyzed by fast-HPLC / ESI-TI. Separation was obtained at 30° C. using a BEH (Waters®) C18 Column (50 mm×2.1 mm, 1.8 μm), by injecting 1 ...
example 2
Extraction and Isolation of the Major Anthocyanins
[0086]A RSWP concentrated extract at 8 CU / kg was extracted using ethyl acetate in order to remove phenolic compounds except anthocyanins. A volume of 30 ml of concentrated extract was diluted in 270 ml of acidified water (pH3) and then washed three times with 300 ml of ethyl acetate. The aqueous phase was concentrated under vacuo.
[0087]The anthocyanin extract obtained was purified on a Sephadex LH2O column (400 mm×260 mm, Pharmacia), being eluted with acetic acid / water (4.6:100, v / v) at a flow rate of 16 ml / h. Two red-colored bands were collected. All fractions collected were analyzed by HPLC and fractions of high purity 85%) were grouped together.
[0088]Analytical HPLC was performed using a LiChrosorb RP-18 Column (250 mm×4.6 mm, 5.0 μm) by injecting 10 pl of the filtered extracts. A combination of two solvents was used for elution: A, Water / HCOOH / Acetonitrile (87 / 10 / 3, v / v / v) and B, Water / HCOOH / Acetonitrile (40 / 10 / 50, v / v / v). The co...
example 3
NMR Analyses
A) Methods
[0090]The NMR experiments (1H, COSY, ROESY, HSQC, HSQC-TOCSY, HMBC, 13C) were obtained at 600.13 MHz on a BRUKER Avance II 600 instrument equipped with a TCI 1H-13C / 15N CryoProbe at 27° C. Dried samples were solubilized in 500 μl in DMSO-d6-TFA-d 99.99% 90:10.
B) Results and Discussion
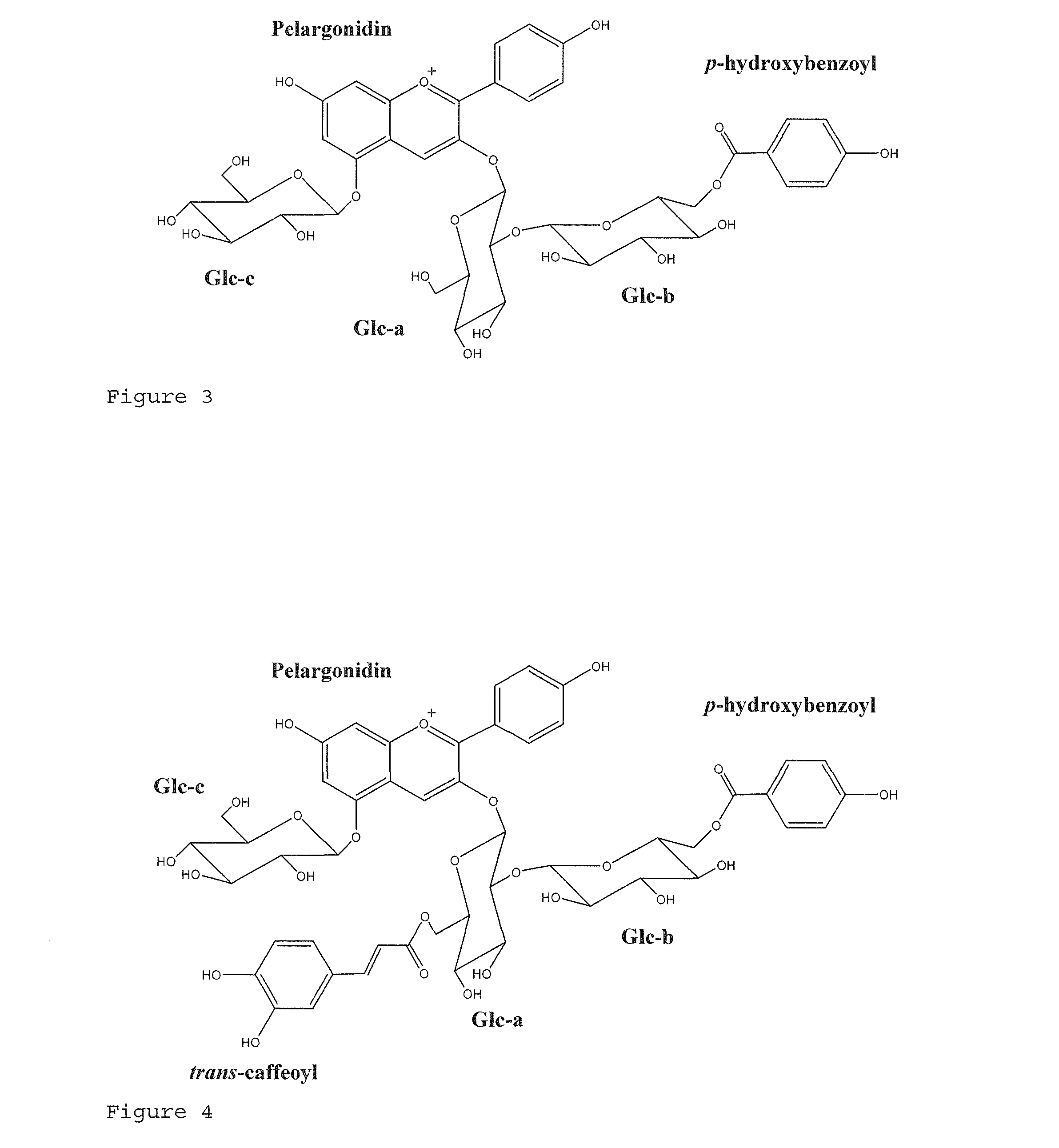
[0091]Anthocyanins 1 and 2 were isolated in sufficient amounts to be characterized by NMR. Their structures were identified by 1H and 13C NMR spectroscopy in DMSO / TFA (90:10). Tables 2 and 3 show the 1H and 13C assignment and HSQC and TOCSY correlations of Anthocyanin 1 and Anthocyanin 2, respectively. Therein un=unresolved, s=singlet, d=doublet and t=triplet.
TABLE 2Anthocyanin 1ATOMδ13Caδ1HbCorrelationsPelargonidin1——2162.7—3144.3—4135.38.94; s ROESY (Glc-a-1)5155.4—6104.36.97; d (1.5)ROESY (Glc-c-1)7168.1—896.47.09; d (1.5)9155.7—10 111.9— 1′119.3— 2′135.38.58; d (8.8) 3′117.17.07; d (8.8) 4′165.4— 5′117.17.07; d (8.8) 6′135.38.58; d (8.8)Glucose-aHSQC-TOCSY (100.1; 80.3; 77.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com