Anode and method of operating an electrolysis cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028]Reference will now be made in detail to the embodiments of the present invention, examples of which are illustrated in the accompanying drawings.
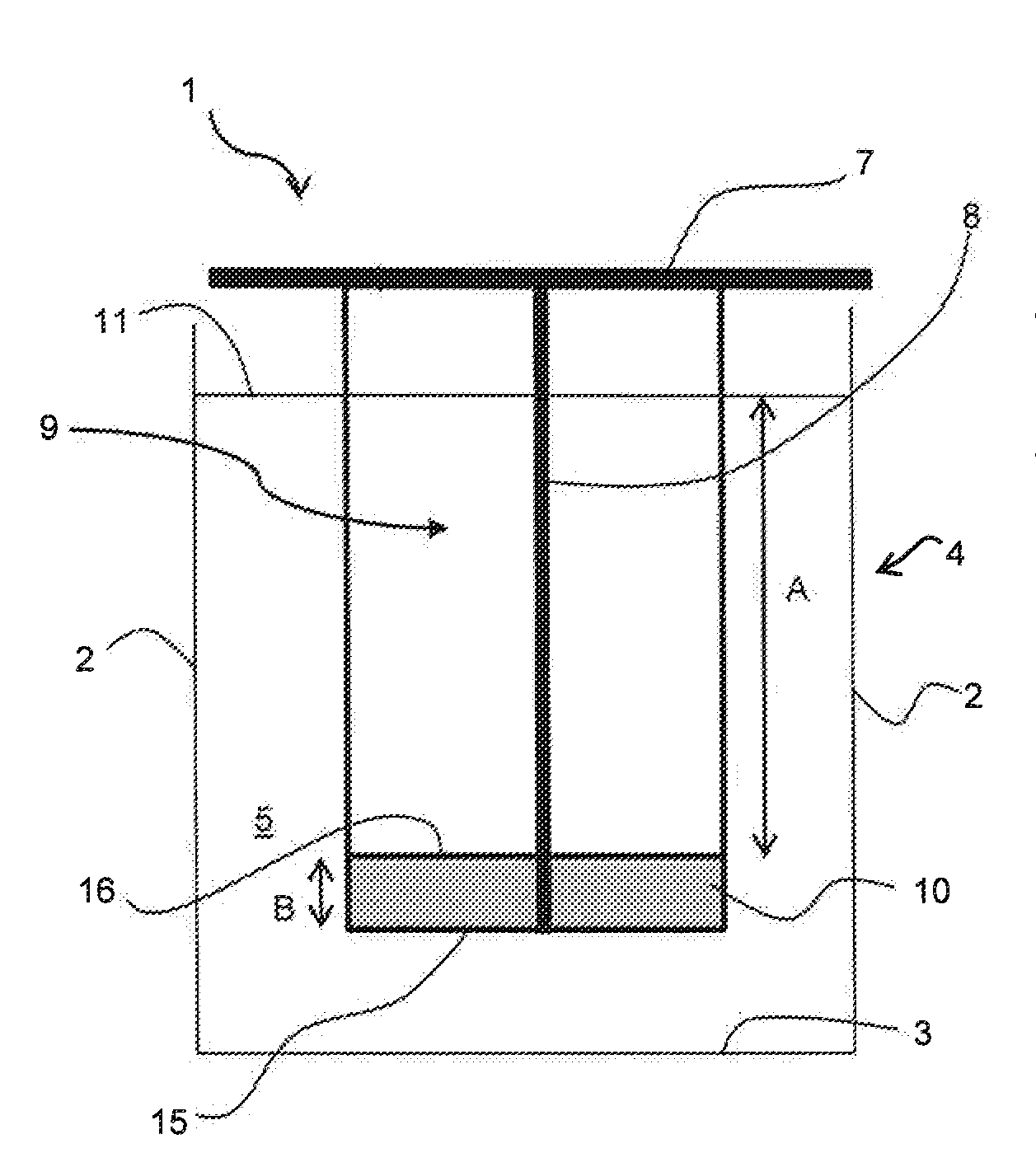
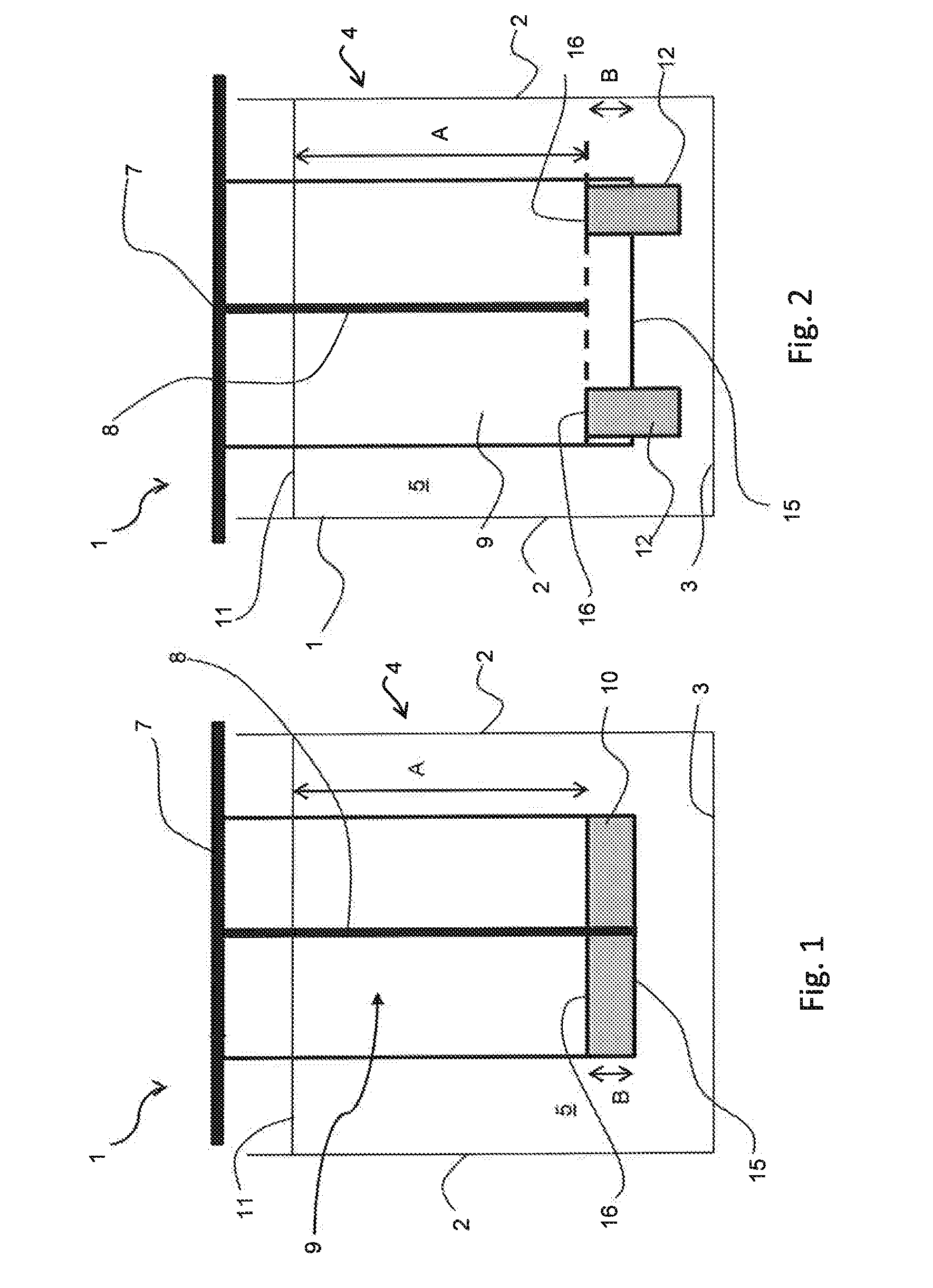
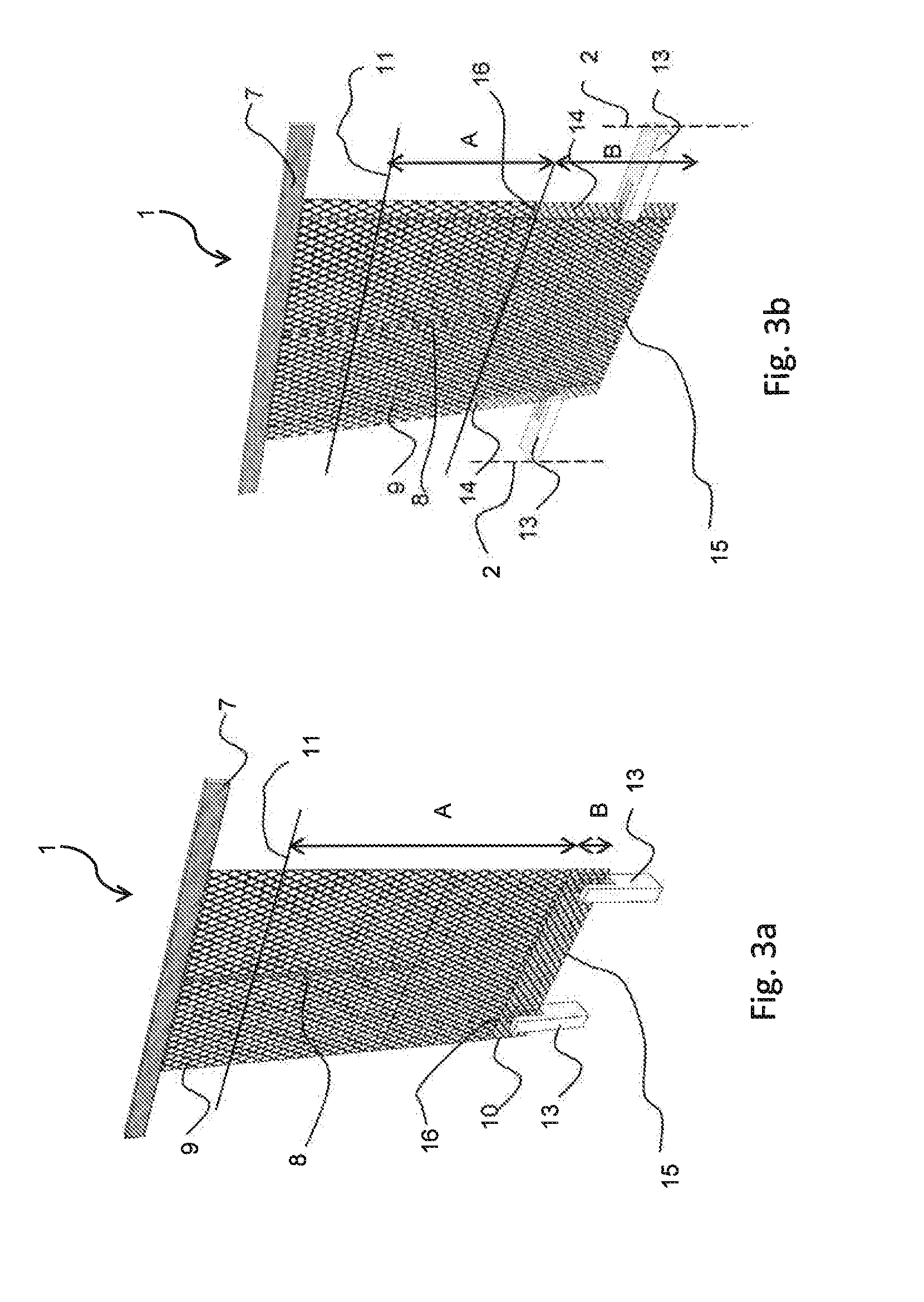
[0029]FIGS. 1 and 2 shows an anode 1 for electrowinning of metals, such as copper in an electrolytic cell 2 having cell walls 3 and cell bottom 4 for holding an electrolyte 5. The anode comprises of a hanger bar 7 for supporting the anode on the conducting rod 8, which distributes the current to the anode, an anode body 9 having at least partly conductive structure allowing the penetration of the electrolyte and an electrocatalytic coating. According to the invention there is arranged a non-conductive element 10, 12, 14 in connection with the anode 1 at a distance A from the electrolyte surface level 11, when the distance A is arranged to be at an interval 0.3-2 meter. This depends on the size of the anode used. The non-conductive element 10, 12, 14 provides means for attaching the anode 1 inside the electrolytic cell 4, which is impo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com