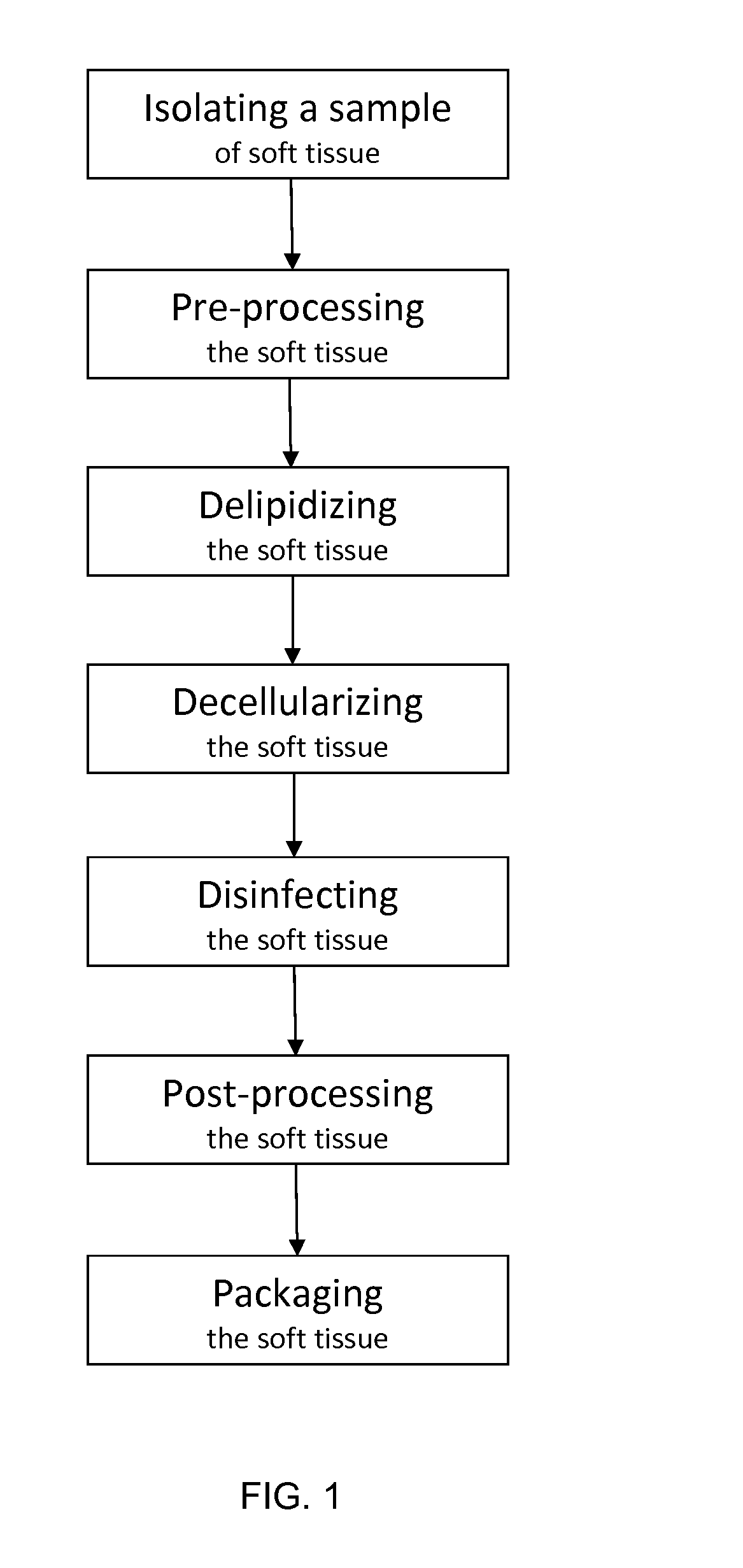
Acellular soft tissue-derived matrices and methods for preparing same
a soft tissue and matrice technology, applied in the field of matrices made from decellularized soft tissues, can solve the problems of tissue rejection, significant risks of biological material transfer from one individual to another,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Decellularization with Sodium Deoxycholate
[0620]Adipose tissue is recovered aseptically from a cadaveric donor. Any muscle, fascia, or other tissues attached to the adipose tissue, if present, are cut away. Equal portions of the adipose tissue are measured into empty flasks and washed by agitation in deionized water, at a ratio of about 1000 ml of water to 500 gm of tissue. After a period of time sufficient to remove blood and loose tissue from the adipose tissue, the water is drained from the flask, while collecting the adipose tissue in a 212 μm to 300 μm sieve. After the water is drained, the adipose tissue is returned to the flask.
[0621]Following the washing step, the adipose tissue is soaked with mechanical agitation in a 4% solution of sodium deoxycholate, at a ratio of about 1000 mL deoxycholate solution to 500 gm of adipose tissue. After a period of time sufficient to disrupt and remove the adipocytes and other cells from the adipose tissue, the deoxycholate solution is drai...
example 2
Decellularization with a Hypertonic Solution
[0624]A sample of adipose tissue is measured into a flask and a hypertonic solution (e.g., 1M NaCl) is added in a 2:1 ratio. The mixture is agitated for at least 12 hours at an ambient temperature. After agitation, the hypertonic solution is decanted, and the adipose tissue is captured in a 212 μm to 300 μm sieve. The recovered adipose tissue is returned to the flask, and soaked in an 0.1% surfactant solution with agitation for at least 12 hours at ambient temperature.
[0625]Following the last water rinse, the adipose tissue is soaked in a sterilizing solution, with agitation. One suitable sterilizing solution would be 0.5% to 1.0% peracetic acid in a mixture of water, ethanol, and propylene glycol. One or more additional soaks in sterilizing solution may be needed to adequately sterilize the adipose tissue. After the final sterilizing soak, the adipose tissue is rinsed repeatedly in deionized water to remove any traces of sterilizing solut...
example 3
Removal of Lipids from an Adipose Tissue
[0627]A sample of adipose tissue, which may be decellularized or untreated, is collected and a size reduction, such as grinding or mincing, is performed. At a temperature of ambient or greater, the adipose tissue is placed in a conical tube or beaker, and homogenized. The homogenized tissue is then centrifuged to separate lipid and water layers from the tissue, taking care not to lose the floating tissue layer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com