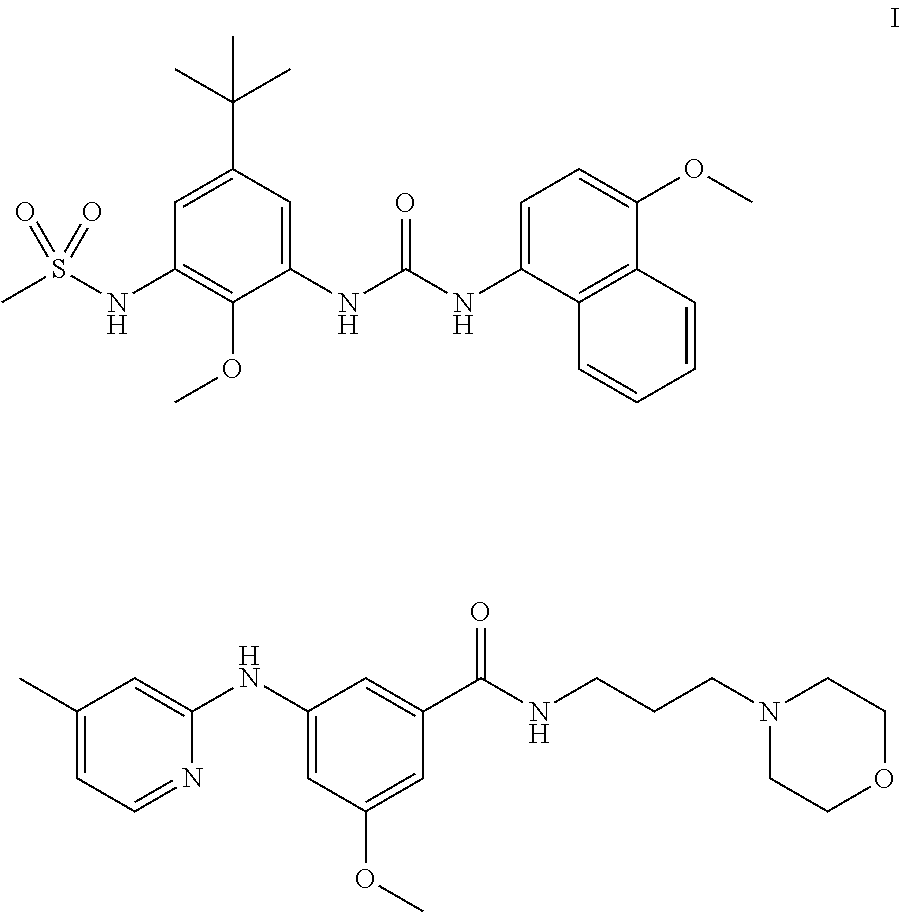
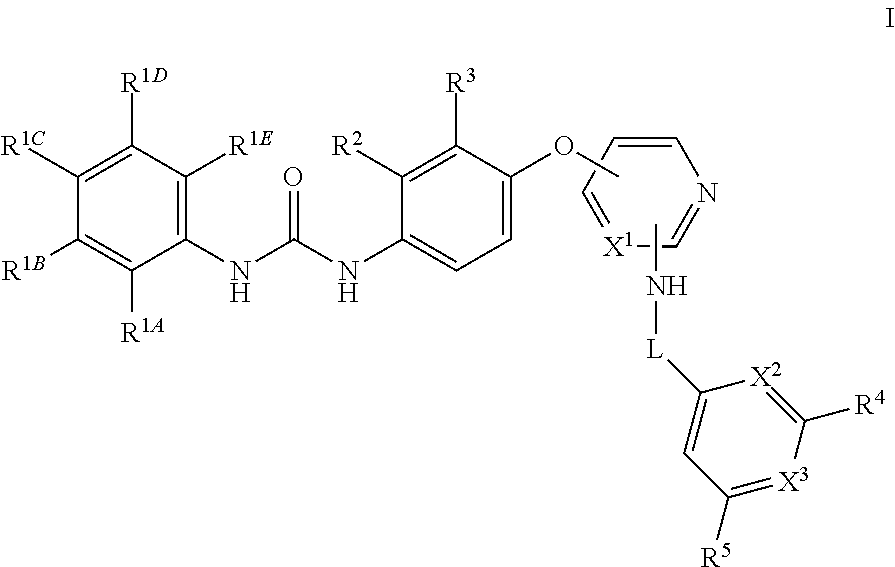
Kinase inhibitors
a kinase inhibitor and inhibitor technology, applied in the field of kinase inhibitors, can solve the problems of high toxicity of inhibitors, hindering the utility of p38 map kinase inhibitors in the treatment of human chronic inflammatory diseases, and patient toxicity, and achieve good anti-inflammatory properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsulfonamido)phenyl)ureido)naphthalen-1-yl)oxy)pyrimidin-2-yl)amino)-5-ethynyl-N-(2-morpholinoethyl)benzamide
[0550]
(i) 3-Amino-5-bromo-N-(2-morpholinoethyl)benzamide
[0551]T3P (50% w / w in EtOAc, 56.2 mL, 94 mmol), was added carefully to a solution of 3-amino-5-bromobenzoic acid (13.6 g, 63.0 mmol), 2-morpholinoethanamine (16.52 mL, 126 mmol) and Et3N (26.3 mL, 189 mmol) in DCM (200 mL). An ice bath was used sporadically to prevent temperature rising above 35° C. Reaction stirred at room temperature for 1 h. Partitioned with sat. aq. NaHCO3 solution (250 mL). Aqueous separated and partitioned with fresh DCM (250 mL). Organics separated, bulked and partitioned with 20% w / w NaCl solution (250 mL). The organic layer was separated, dried (MgSO4), filtered and solvent evaporated. The crude product was dissolved in DCM (100 mL) and the sub-title compound (13 g) crystallised out on standing as a light tan crystalline solid.
[0552]1H NMR (400 MHz,...
example 2
3-Ethynyl-5-((4-((4-(3-(3-fluoro-5-morpholinophenyl)ureido)naphthalen-1-yl)oxy)-pyrimidin-2-yl)amino)-N-(2-morpholinoethyl)benzamide
[0572]
(i) Phenyl(3-fluoro-5-morpholinophenyl)carbamate
[0573]A stirred suspension of 3-fluoro-5-morpholinoaniline (1.00 g, 5.10 mmol) and sodium bicarbonate (0.878 g, 10.45 mmol) in DCM (10 mL) and THF (4 mL) was treated dropwise with phenyl chloroformate (0.7 mL, 5.57 mmol). After ˜0.1 mL had been added the mixture became very thick and difficult to stir, so it was diluted with more DCM (5 mL) and THF (2 mL) and stirred overnight. The mixture was treated with more sodium bicarbonate (0.086 g, 1.019 mmol) and phenyl chloroformate (0.07 mL, 0.557 mmol) and stirred over the weekend. The mixture was diluted with DCM (30 mL), was washed with water (30 mL) and filtered through a phase-separating cartridge. The filtrate was evaporated and the residue was purified on a 40 g redisep silica cartridge using a gradient of 0 to 50% of ethyl acetate in isohexane as e...
example 3
3-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsulfonamido)phenyl)ureido)naphthalen-1-yl)oxy)pyridin-2-yl)amino)-5-methoxy-N-(2-(2-(2-methoxyethoxyl)ethoxy)ethyl)benzamide
[0586]
(i) 3-Amino-5-methoxy-N-(2-(2-(2-methoxyethoxy)ethoxy)ethyl)benzamide
[0587]3-Amino-5-methoxybenzoic acid (1.0 g, 5.98 mmol) was added to an ice cold suspension of 2-(2-(2-methoxyethoxyl)ethoxy)ethanamine (1.2 g, 7.35 mmol), 50% T3P in ethyl acetate (4.50 mL, 7.56 mmol) and TEA (2.5 mL, 17.94 mmol) in ethyl acetate (15 mL). The mixture was allowed to warm to rt and stir overnight. Saturated aq. NaHCO3 solution (20 mL) was added and the mixture was extracted with ethyl acetate (3×10 mL). The combined organic phases were washed with saturated brine (20 mL), dried (MgSO4) and concentrated under reduced pressure to yield a yellow oil. The oil was purified by chromatography on the Companion (40 g column, 0-100% acetone / toluene) to afford a pale yellow oil. The oil was purified by chromatography on the Companion (4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass mean aerodynamic diameter | aaaaa | aaaaa |
| mass mean aerodynamic diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com