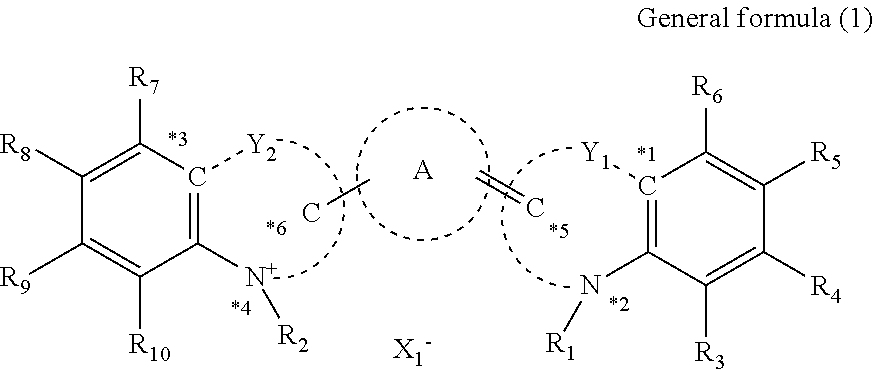
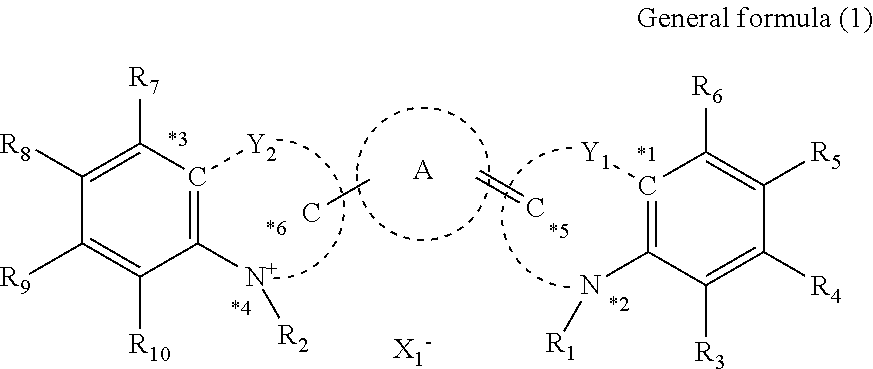
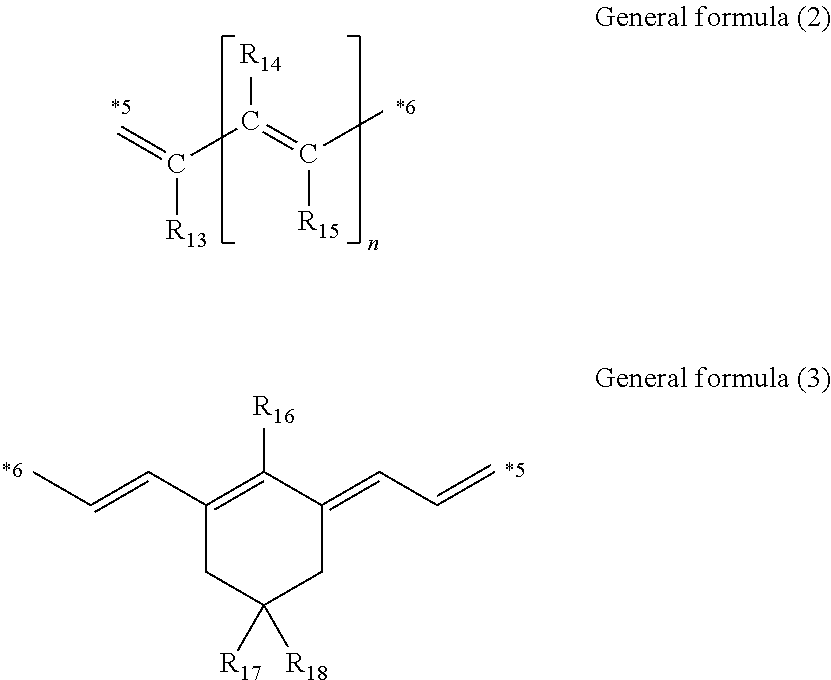
Cancer cell inhibitory drug and cancer stem-cell detection probe
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0159]Production Examples of the compounds of the present invention will be shown.
[0160]Production of Compound (1)
Under a nitrogen atmosphere, to a solution of compound (A1) (0.61 g (2.0 mmol)) in anhydrous acetic acid (10 mL), a compound (B1) (0.20 g (1.0 mmol)) and anhydrous sodium acetate (0.16 g (2.0 mmol)) were added and stirred at 100° C. for one hour. After completion of the reaction, while the reaction solution was cooled, saturated saline (100 mL) was gently added dropwise to cool the reaction solution to room temperature. Furthermore, the reaction solution was extracted twice with dichloromethane (50 mL) and dried over anhydrous sodium acetate. Thereafter, the organic layer was concentrated under reduced pressure. The residue was purified by silica gel chromatography and the purified product was recrystallized from diethyl ether to obtain the compound (1) (0.29 g (yield 59%)). The desired product was confirmed by 1H nuclear magnetic resonance spectroscopic analysis (ECA-40...
example 2
Measurement of Fluorescent Property of Compound
[0164]A 5 μM DMSO solution of each of the compounds shown in the following Table 1 was prepared. The excitation wavelength and fluorescence wavelength of the compound were measured by a FL4500 spectrofluorometric measuring machine manufactured by Hitachi High-Technologies Corporation.
TABLE 1ExcitationFluorescenceCompoundwavelengthλexwavelength λemCompound 1485575Compound 4563569Compound 5560628Compound 7344381Compound 10586615Compound 11354469Compound 14491510Compound 16474509Compound 17492511Compound 18492510Compound 20516602Compound 21473564Compound 22553570Compound 24496569Compound 26684710Compound 27650675Compound 28589614Compound 30643662Compound 34638661Compound 35571620Compound 37650770Compound 40819825Compound 43797816Compound 45665681Compound 46679715Compound 47615637Compound 49830831Compound 50831833Compound 54774800Compound 55688715Compound 59804520Compound 60670696
example 3
Confirmation on Cancer Cell Inhibitory (Growth Suppressive) Action Against Pancreatic Cancer Cells
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inhibition | aaaaa | aaaaa |
| Luminescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com