Process for improved opioid synthesis
a technology of opioid synthesis and process, which is applied in the direction of biocide, drug composition, chemical production, etc., can solve the problems of inability to approve a pharmaceutical composition or dosage form for use and sale, difficulty in separating by-products from final opioids, and inability to reduce the number of by-products, etc., to achieve volume efficient, reduce manufacturing costs, and reduce by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 14-hydroxycodeinone sulfate
[0541]
[0542]14-hydroxycodeinone sulfate was prepared as follows:
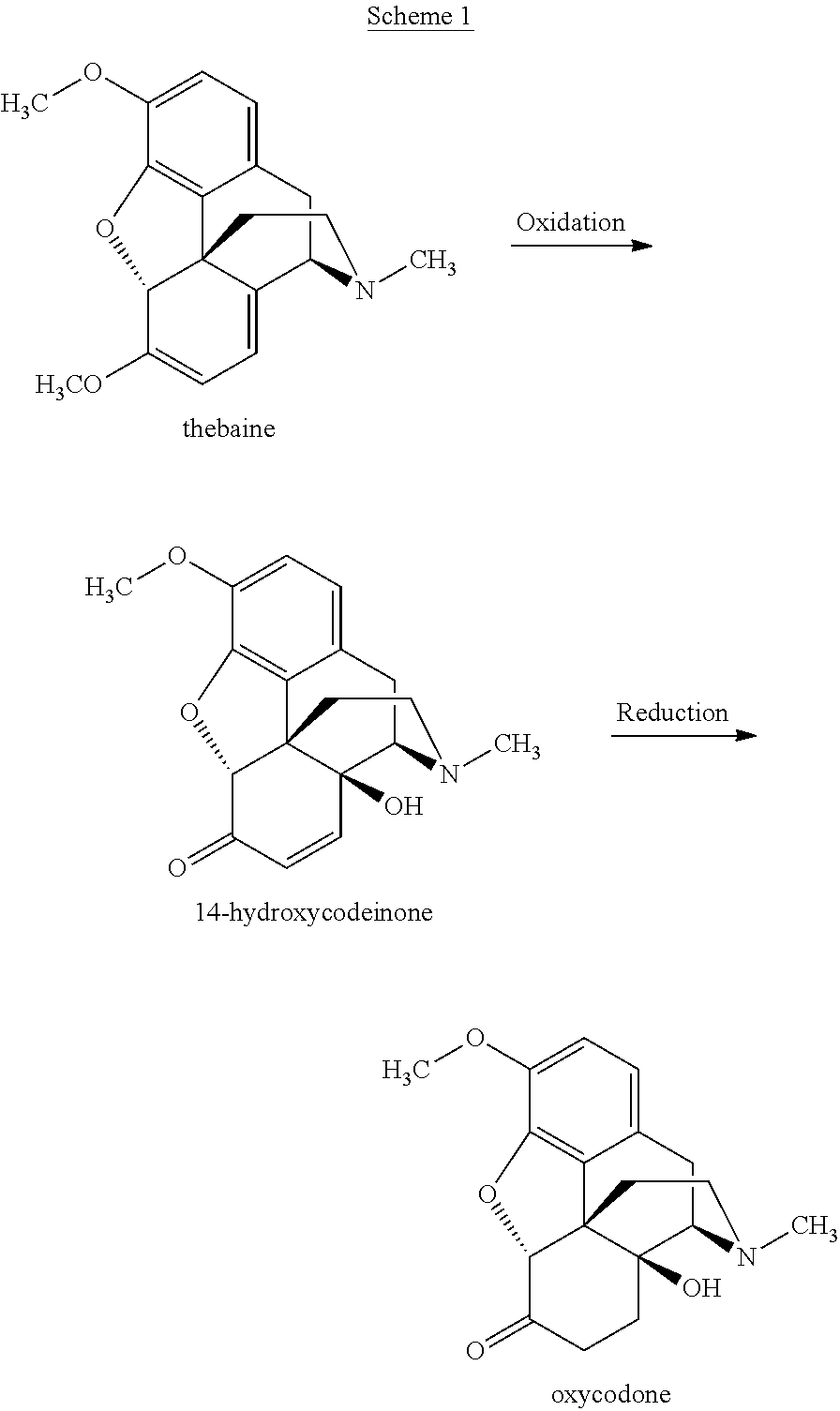
[0543]1. Into a 100 mL jacketed vessel equipped with a temperature probe, overhead stirrer and an addition funnel, thebaine (12.0 g, 38.6 mmol) was charged as a slurry in deionized water (18 mL).
[0544]2. The jacket temperature for the vessel was set to 20° C. and the slurry was stirred at 300 rpm.
[0545]3. 88% formic acid (6 mL, 140 mmol) was added into the reaction mixture. The solids readily dissolved into solution upon this addition. During the formic acid addition, the temperature of the reaction mixture increased to 29° C.
[0546]4. Sulfuric acid (1.15 mL, 21 mmol) was added to the solution, and the solution was stirred at 300 rpm.
[0547]5. After the solution temperature had cooled below 22° C., 35% hydrogen peroxide (4.00 mL, 46.5 mmol) was added to the reaction over 15 minutes, using the addition funnel.
[0548]6. After the peroxide addition was complete, an additional 3 mL of ...
example 2
Preparation of Oxycodone Base
[0560]
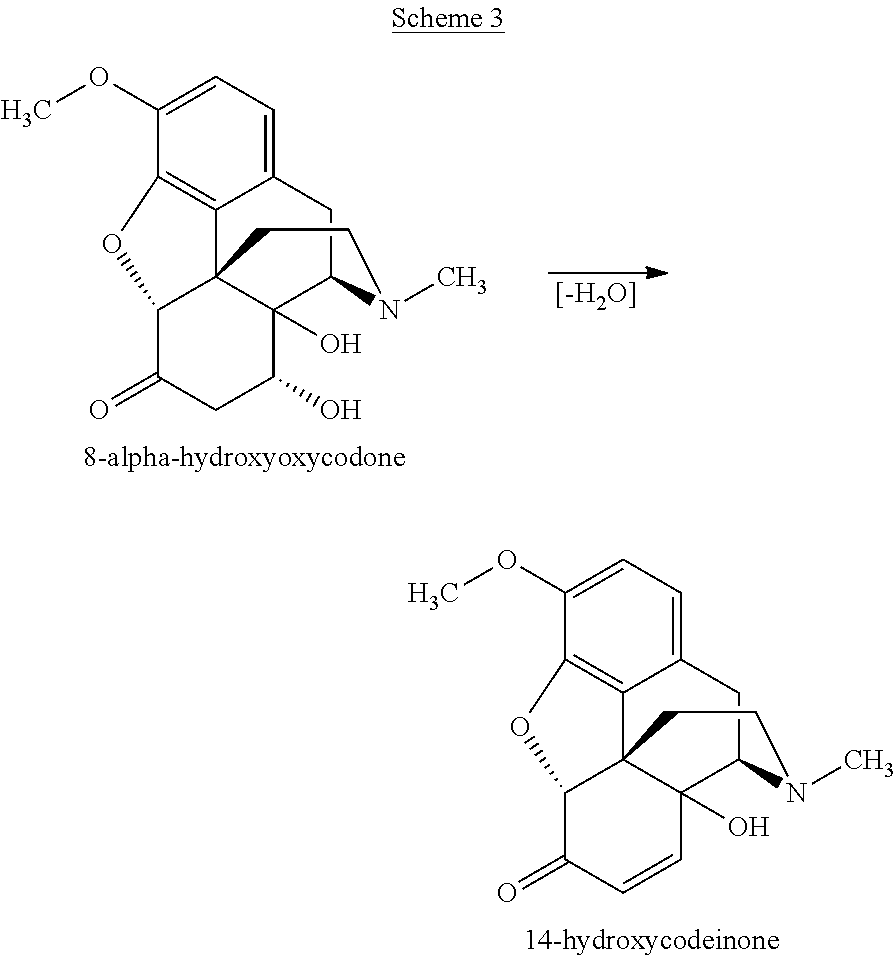
[0561]A composition comprising 99.13% of oxycodone base, 26 ppm 14-hydroxycodeinone, 565 ppm of 8α-hydroxyoxycodone and 298 ppm of 8β-hydroxyoxycodone, based on HPLC area percent, was prepared as follows:
[0562]1. Into a 300 mL hydrogenation vessel equipped with a magnetic stir bar, 14-hydroxycodeinone sulfate obtained in Example 1 (9.01 g, 12.43 mmol (calculated without water of crystallization)), deionized water (90 mL) and methanol (40 mL) were charged. The majority of solids dissolved into solution.
[0563]2. Formic acid (1.20 mL, 28.0 mmol) and 5% palladium on carbon (0.065 g) were added into the reaction mixture.
[0564]3. The vessel was sealed, stirred at 750 rpm and heated to 40° C.
[0565]4. The mixture was then hydrogenated at 60 psia (413.69 kPa) for 5 hours.
[0566]5. The reaction was vented, purged with nitrogen, vented and hydrogenated at 60 psia (413.69 kPa) for an additional 1 hour.
[0567]6. The reaction was vented, purged with nitrogen and c...
example 3
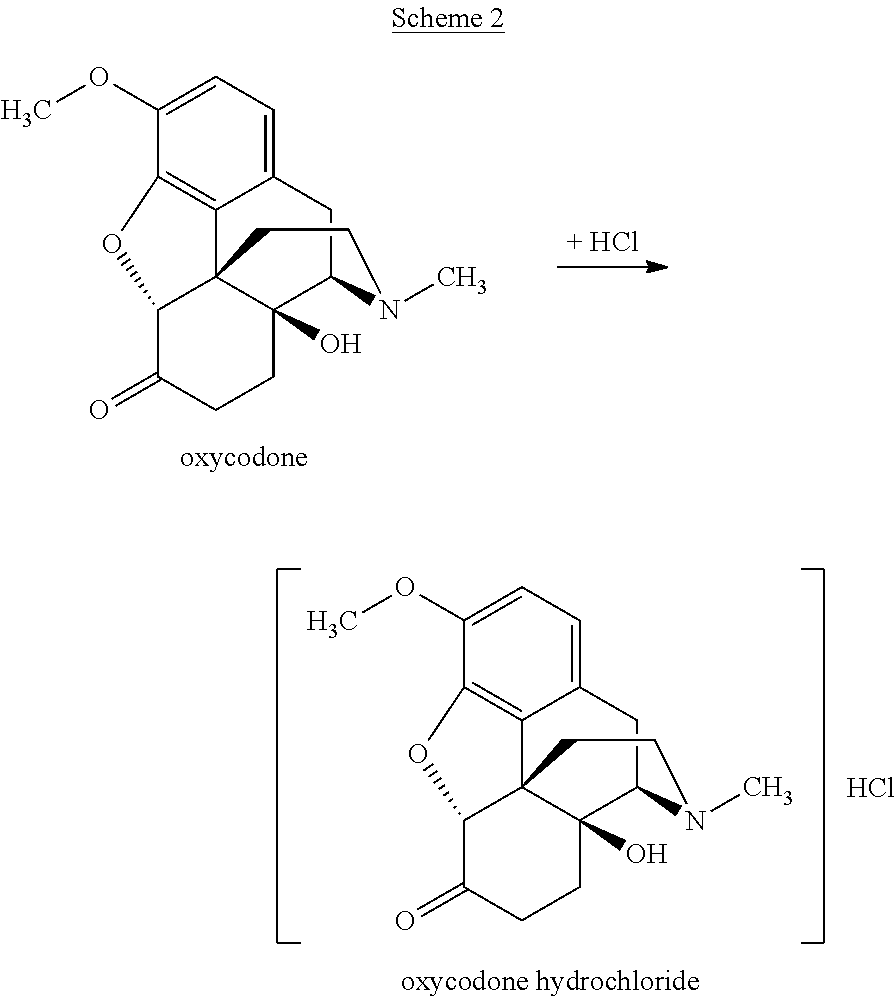
Preparation of Oxycodone Hydrochloride
[0575]
[0576]A composition comprising 99.72% of oxycodone hydrochloride, 9 ppm of 14-hydroxycodeinone hydrochloride, 103 ppm of 8α-hydroxyoxycodone hydrochloride, and 191 ppm of 8β-hydroxyoxycodone hydrochloride, based on HPLC area percent, was prepared as follows:
[0577]1. Into a 300 mL jacketed reaction vessel equipped with a temperature probe, reflux condenser, and overhead stirrer, was charged oxycodone (4.01 g, 12.0 mmol), deionized water (6 mL) and isopropanol (45 mL). The oxycodone comprised 99.13% of oxycodone, 26 ppm of 14-hydroxycodeinone, 565 ppm of 8α-hydroxyoxycodone, and 298 ppm of 8β-hydroxyoxycodone.
[0578]2. The mixture was stirred at 250 rpm and the external jacket of the vessel was heated to 80° C.
[0579]3. When the internal temperature of the mixture had reached 40° C., 37% hydrochloric acid (1.05 mL, 12.8 mmol) was added to the reaction vessel.
[0580]4. When the internal temperature of the mixture had reached 74° C., all visible ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| threshold | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com