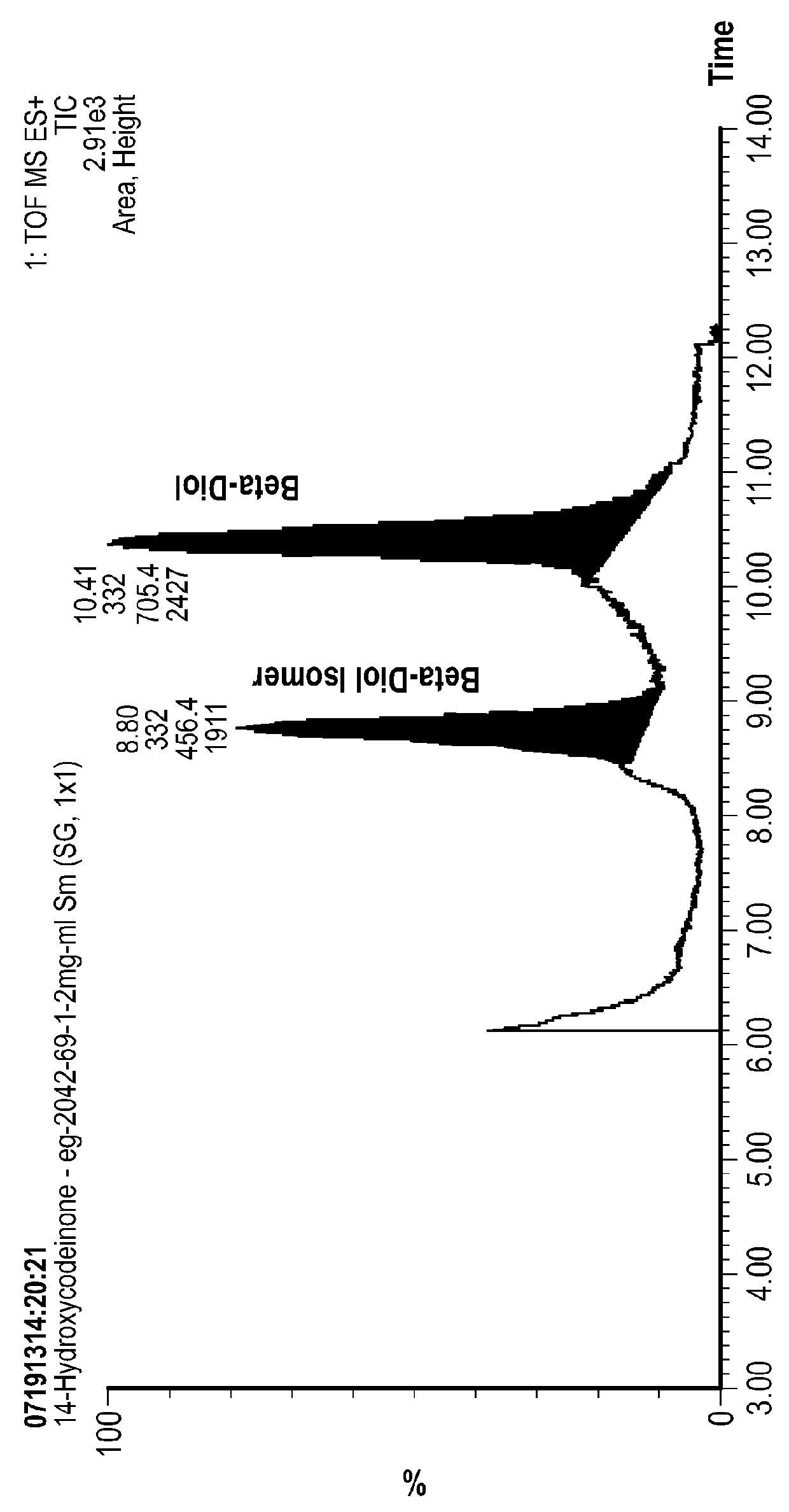
Processes for Making Opioids Including 14-Hydroxycodeinone and 14-hydroxymorphinone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparative
[0084]A 500 mL jacketed reaction vessel is charged with 85% formic acid (26.1 g, 480 mmol, 15.0 equivalents) and then thebaine (12.5 g wet, 10 g dry, 32 mmol) is added while stirring and maintaining the temperature at <35° C. The resulting solution is cooled to 25-30° C. and then 30% w / w hydrogen peroxide solution in water (4.0 g, 35.2 mmol, 1.1 equivalents) is added over 30-45 minutes while maintaining the reaction temperature in the 25-30° C. range. The reaction mixture is stirred at 25-30° C. for 9 h, then cooled to 0-10° C. and held for 11 h.
[0085]A 1:1 v / v mixture of 28-30% ammonium hydroxide solution and water (˜64 mL) is next added to adjust the pH to 9.3 (target 8.5-9.5) while maintaining the temperature at <30° C. The resulting mixture is stirred at 0-10° C. for 30 min and a product (a white sticky solid) is filtered, washed with water (30 mL) and then ethanol (20 mL). The wet product is allowed to dry on the filter under applied vacuum for 6 min to afford a crud...
example 2
[0086]A 500 mL jacketed reaction vessel is charged with 85% formic acid (26.1 g, 480 mmol, 15.0 equivalents) and then thebaine (12.5 g wet, 10 g dry, 32 mmol)) is added while stirring. The resulting solution is stirred, the temperature is adjusted to 25-30° C. and sulfuric acid (1.26 g, 0.7 mL, 12.8 mmol, 0.4 equivalents) is added cautiously with the batch temperature increasing from 27° C. to 33° C. A 30% w / w hydrogen peroxide solution in water (4.0 g, 35.2 mmol, 1.1 equivalents) is next added over 1.5 h while maintaining the reaction temperature in the range 25-30° C. The reaction mixture is stirred at 25-30° C. for 10 h, then cooled to 0-10° C. and held for 10 h.
[0087]A 1:1 v / v mixture of 28-30% ammonium hydroxide solution and water (70 mL) is next added to adjust the pH to 9.2 (target 8.5-9.5) while maintaining the temperature at <30° C. The resulting mixture is stirred at 0-10° C. for 1.5 h and a solid product is filtered, washed with water and then methanol. The wet product is...
example 3
[0088]A 1 L reaction vessel is charged with 85% formic acid (26.1 g, 480 mmol, 15.0 equivalents) and then thebaine (12.5 g wet, 10 g dry, 32 mmol)) is added while stirring. The resulting solution is stirred and the temperature is adjusted to 25-30° C. and trifluoroacetic acid (2.9 g, 1.9 mL, 25.7 mmol, 0.8 equivalents) added cautiously. A 30% w / w hydrogen peroxide solution in water (4.0 g, 35.2 mmol, 1.1 equivalents) is next added over 1.25 h. The reaction mixture is stirred at 25-30° C. for 8 h, then cooled to 0-5° C. and held for 8 h.
[0089]A 1:1 v / v mixture of 28-30% ammonium hydroxide solution and water is next added to adjust the pH to within 8.5-9.5 range while maintaining the temperature at <30° C. The resulting mixture is stirred at 0-5° C. for 2 h and a solid product is filtered, washed with water and then methanol. The wet product is allowed to dry on the filter under applied vacuum to afford crude 14-hydroxycodeinone (wet product weight 15.9 g, dry product weight 8.90 g, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com