Glycolic acid polymers and method of producing the same
a polyglycolic acid and polymer technology, applied in the field of polymer, can solve the problems of difficult preparation of pure glycolide which yields high molecular weight polyglycolic acid, loss of desired optical purity of the precursor, and low molar mass of polymer in the process, so as to achieve high molecular weight and increase the molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
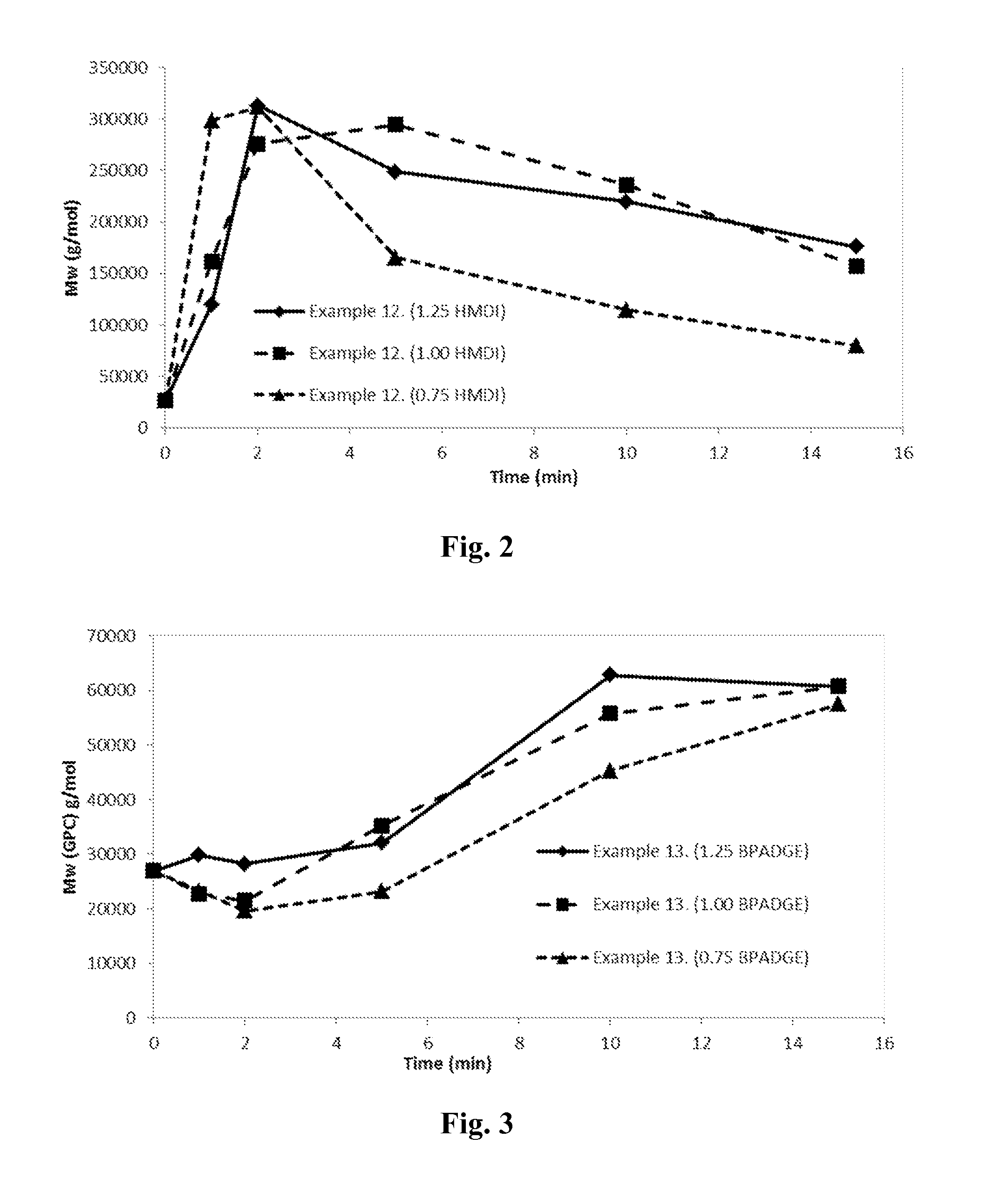
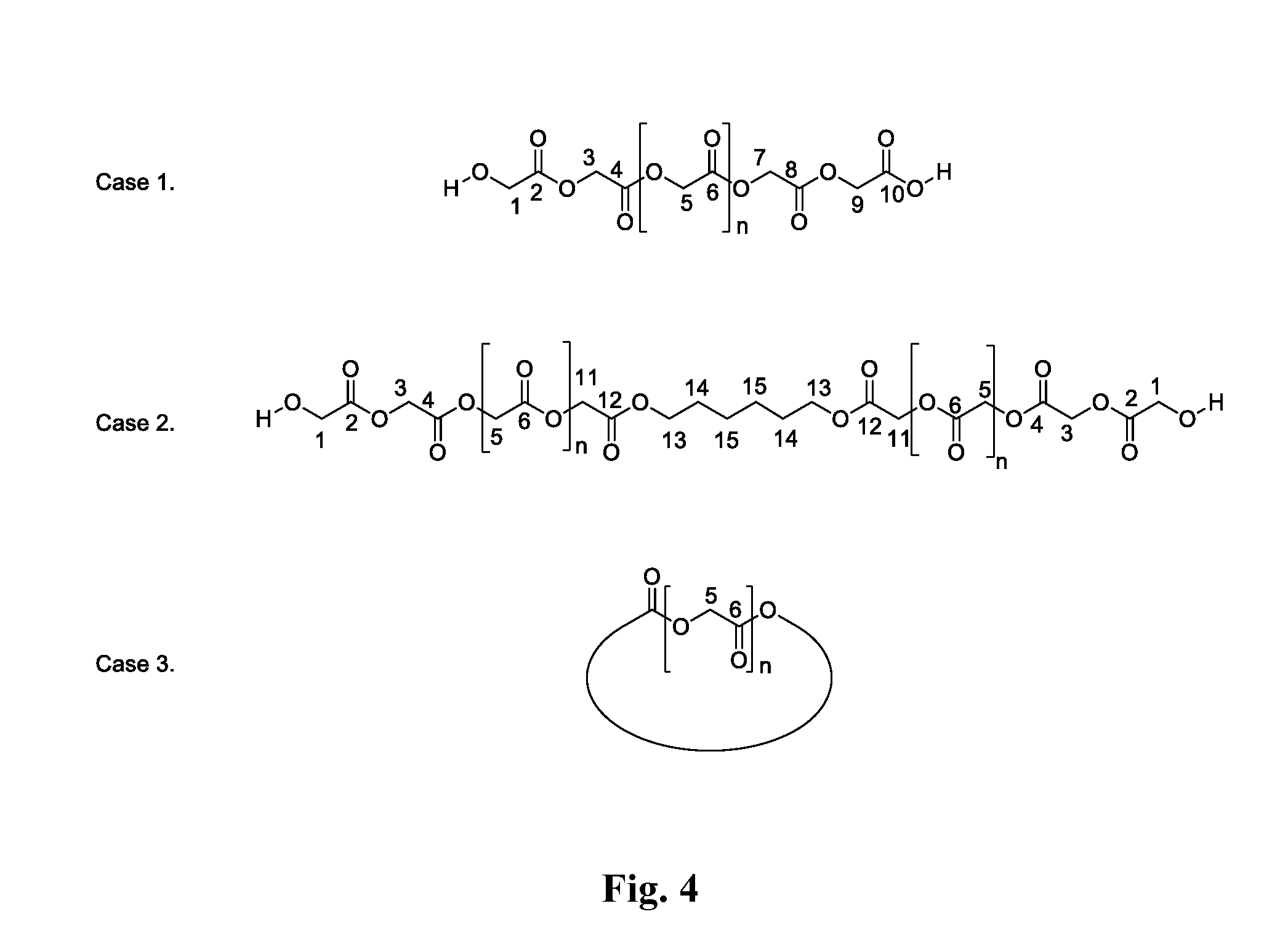
Examples
example 1
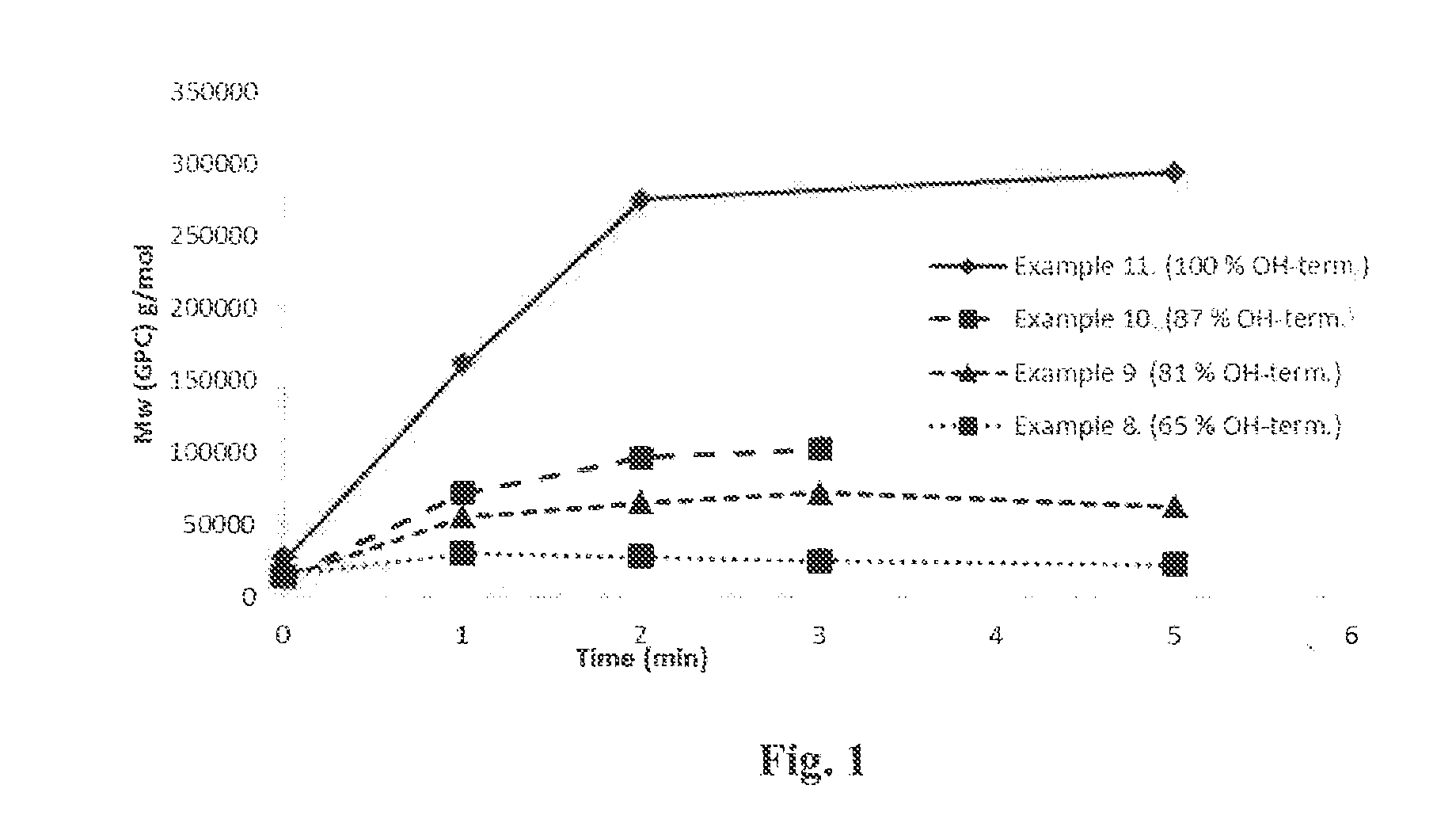
[0111]500 g solid glycolic acid, 15.6 g hexanediol (2 mol-%) and 0.26 g SnOct2 (0.05 m-%) were added to a 1000 mL flask connected to a rotavapor and an oil bath. Temperature was increased gradually from 130° C. to 190° C. and pressure was decreased gradually from 500 mbar to 30 mbar during four hours. When target temperature and pressure were achieved, reaction was continued for 24 hours. Temperature was increased to 230° C. and reaction was continued for two hours. Yield 392 g, Mn (NMR) 2 000 g / mol, Mn (GPC) 10 800 g / mol, Mw (GPC) 15 000 g / mol, Tg 24° C., Tc 90° C., ΔHc 15 J / g, Tm 209° C., ΔHm −99 J / g, 65% OH-terminated.
example 2
[0112]500 g solid glycolic acid, 23.3 g hexanediol (3 mol-%) and 0.26 g SnOct2 (0.05 m-%) were added to a 1000 mL flask and reaction was performed similarly as in example 1. Yield 403 g, Mn (NMR) 1 500 g / mol, Mn (GPC) 10 000 g / mol, Mw (GPC) 14 400 g / mol, Tg 19° C., Tc 95° C., ΔHc 51 J / g, Tm 203° C., ΔHm −95 J / g, 81% OH-terminated.
example 3
[0113]500 g solid glycolic acid, 31.2 g hexanediol (4 mol-%) and 0.27 g SnOct2 (0.05 m-%) were added to a 1000 mL flask and reaction was performed similarly as in example 1. Yield 410 g, Mn (NMR) 1 300 g / mol, Mn (GPC) 10 300 g / mol, Mw (GPC) 14 400 g / mol, Tg 14° C., Tc 88° C., ΔHc 47 J / g, Tm 196° C., ΔHm −97 J / g, 87% OH-terminated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of crystallinity | aaaaa | aaaaa |
| degree of crystallinity | aaaaa | aaaaa |
| degree of crystallinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com