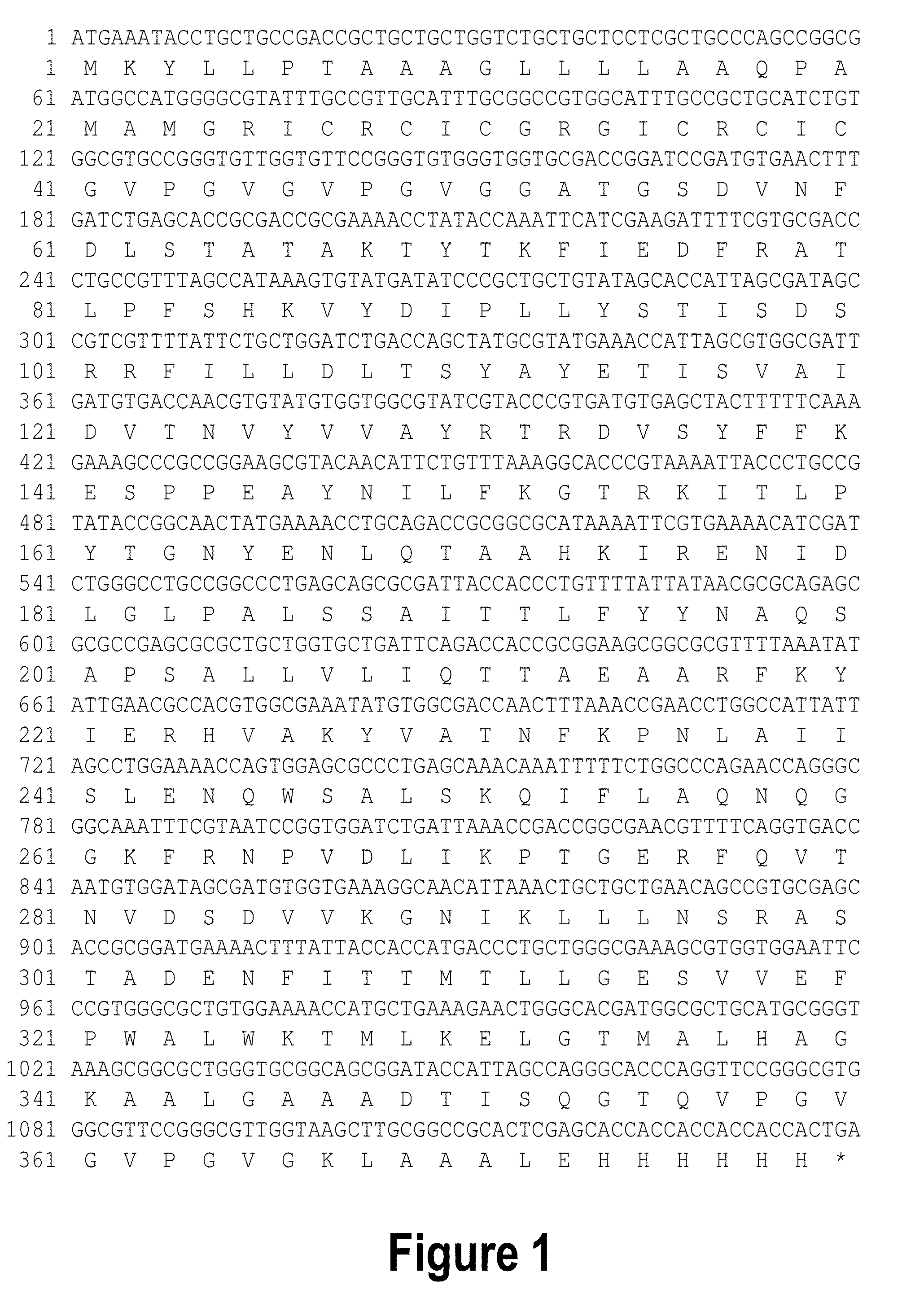Antimicrobial fusion compounds and uses thereof
a technology of fusion compounds and antimicrobials, applied in the field of antimicrobial fusion compounds, can solve the problems of drug loss, high morbidity around the world, and growing problem of microbial resistance to such agents, and achieve the effect of optimal effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0139]Construction and Design of Expression Vector
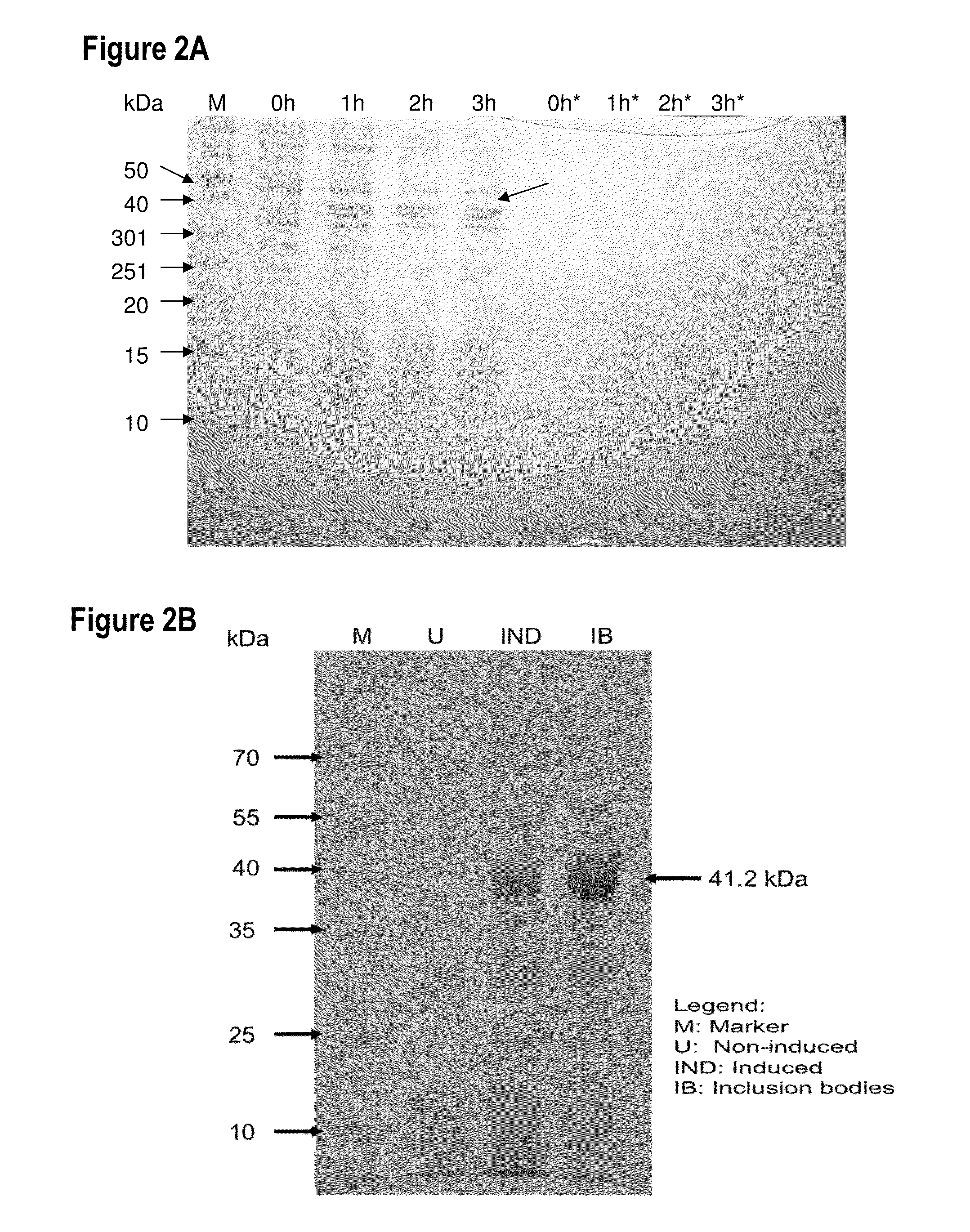
[0140]The gene encoding RetroMAD1 A-B-C with SEQ ID NO:1 was synthesized and cloned into backbone of vector pGA4 at the KpnI / SacI site by contract service (GeneArt AG, Germany). The expected product size was 1140 bp, which encoded a 379 amino acid and an expected size of 41.2 kDa. The polynucleotide sequence and the translated polypeptide sequence are shown in FIG. 1. The gene was sub-cloned into a pET expression vector (Novagen), pET-26(b) at the NcoI / HindIII sites. Kanamycin was used as a marker for selection and maintenance of culture purposes. This vector was inducible under the addition of isopropyl-beta-D-thiogalactopyranoside (IPTG). The plasmid, pRMD1 was then transformed into BL21(DE23) cells (Novagen) and plated on a selective media with Kanamycin.
[0141]Expression of RetroMAD1 from E. coli
[0142]One recombinant clone was grown in 10 ml of LB Bertani (DIFCO) medium, supplemented with 30 μg / ml kanamycin, at 37° C. overnight. ...
example 2
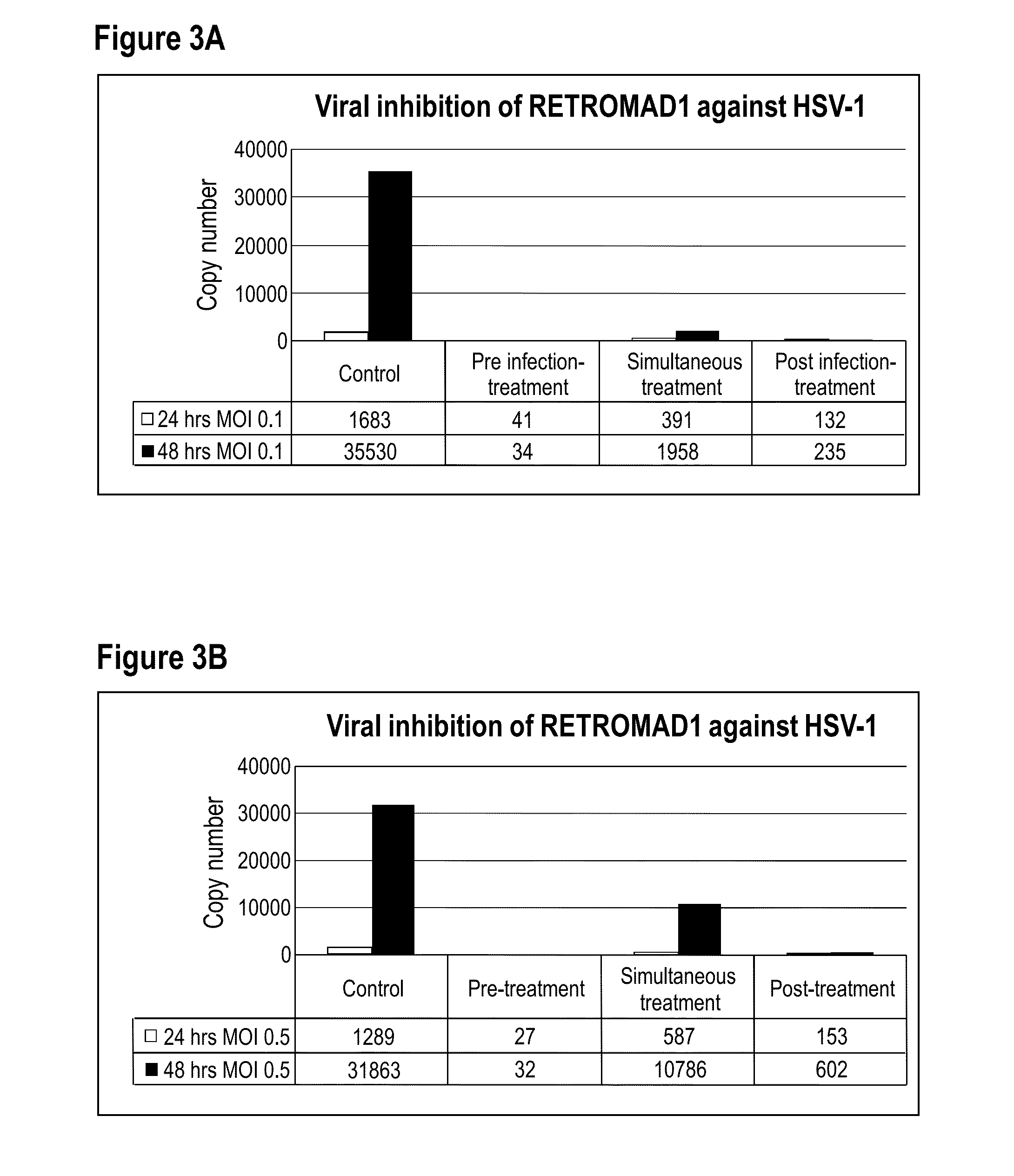
[0149]Virus Stocks
[0150]The herpes simplex virus type-1 (HSV-1) and type-2 (HSV-2) were obtained from Medical Microbiology Department in the Faculty of Medicine, University of Malaya. The virus stock was prepared by inoculating monolayer of vero cells in a 25-cm2 tissue culture flask with virus diluted 1:5 to 1:10 in 1 mL of Dulbecco's Modified Eagle Medium (DMEM) (HyClone) containing 2% Fetal bovine serum (FBS) (HyClone). The flask was placed in an incubator at 37° C. to allow virus adsorption. After 1 hour, 4 mL of DMEM supplemented with 2% FBS was added and the cells were allowed to continue propagating at 37° C. for 6 to 7 days until the cytophatic effect (CPE) are confirmed. Cell debris was removed by centrifugation at 1,500×g for 5 minutes. The viral supernatant was collected in aliquots of 1 mL each and stored at −80° C. until further use.
[0151]Virus Titration by Plaque Assay
[0152]The virulence expressed as plaque forming unit per millilitre (PFU / ml), of each HSV type was tit...
example 3
[0160]Virus Stocks
[0161]The dengue virus type-1 (DENY-1), type-2 (DENV2), type-3 (DENV-3) and type-4 (DENV-4) strain used in this study is a prototype of Hawaii, New Guinea C, H87 and H241 strain respectively (courtesy of Medical Microbiology Department in the Faculty of Medicine, University of Malaya). The virus stock was prepared by inoculating monolayer of C6 / 36 cells in a 25-cm2 tissue culture flask with virus diluted 1:5 to 1:10 in 1 mL of Leibovitz's L-15 containing 2% FBS. The flask was placed in an incubator at 28° C. to allow virus adsorption. After 1 hour, 4 mL of Leibovitz's L-15 supplemented with 2% FBS was added and the cells were allowed to continue propagating at 28° C. for 6 to 7 days until the cytophatic effect (CPE) are confirmed. Cell debris was removed by centrifugation at 1,500×g for 5 minutes. The viral supernatant was collected in aliquots of 1 mL each and stored at −80° C. until further use.
[0162]Virus Titration by Plaque Assay
[0163]The virulence expressed as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com