Vehicle for delivering a compound to a mucous membrane and related compositions, methods and systems
a technology for delivering compounds and mucous membranes, applied in drug compositions, blood/immune system cells, immunological disorders, etc., can solve the problems of alarmingly increasing incidence of diseases, challenging system and immunomodulatory properties, and challenging effective delivery of desired compounds or substances to mucous membranes, etc., to suppress immunity, reduce dendritic cell activation, and inhibit t cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Immunomodulatory Capsular Polysaccharide PSA is Actively Sorted into OMVs of B. fragilis
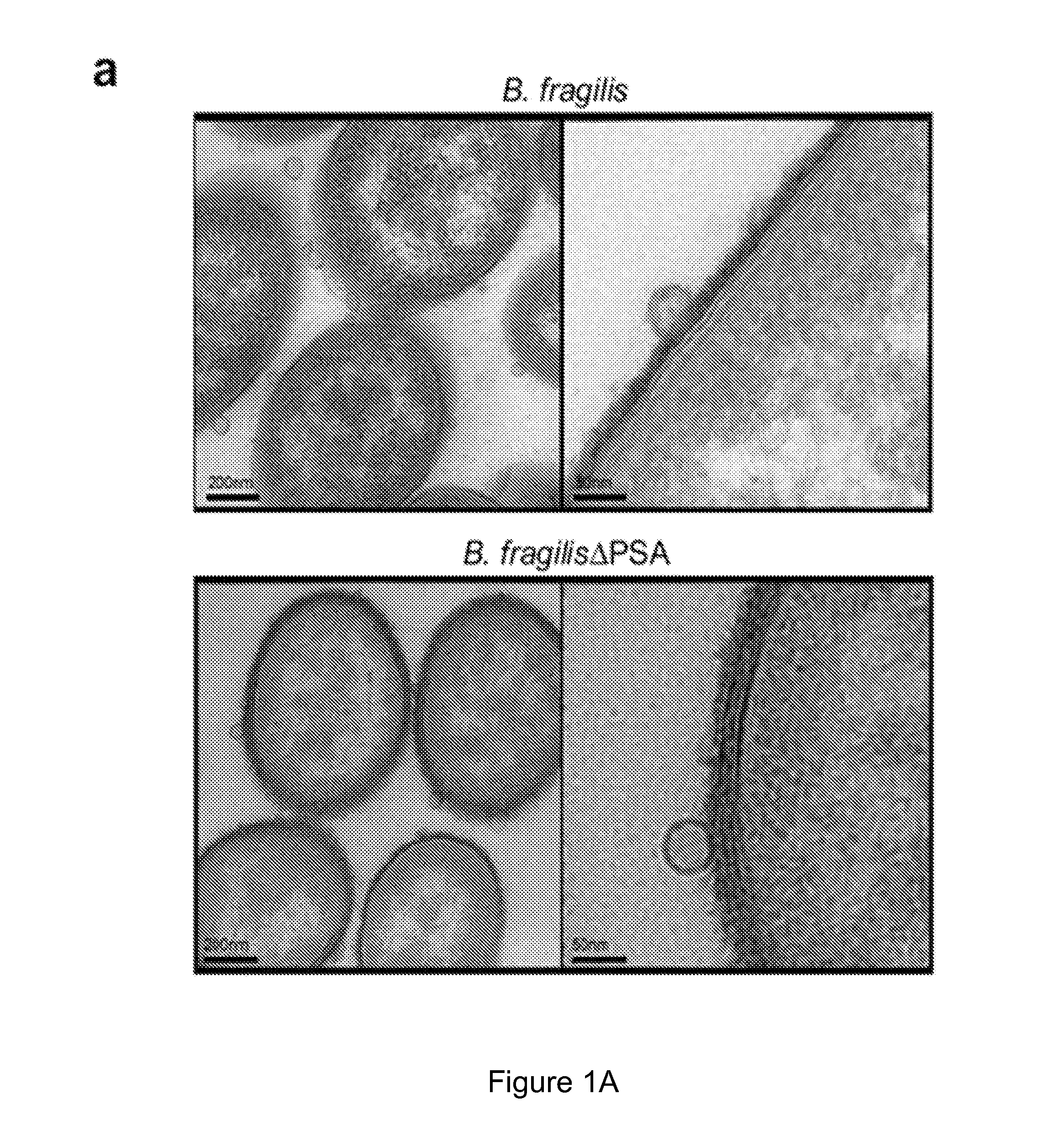
[0176]Ultrathin sections of EDL-enriched B. fragilis were prepared as described in materials and methods and imaged by transmission electron microscopy.
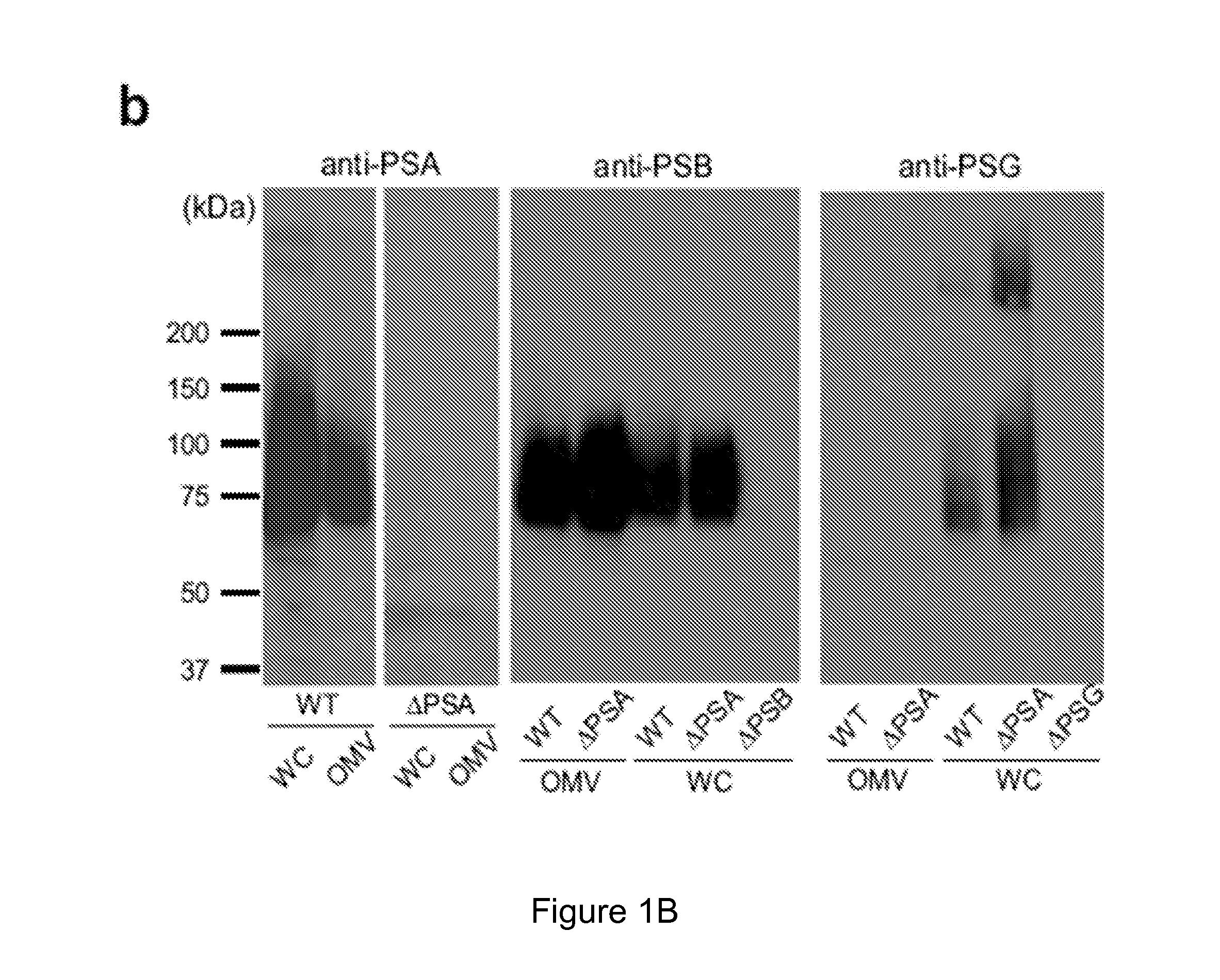
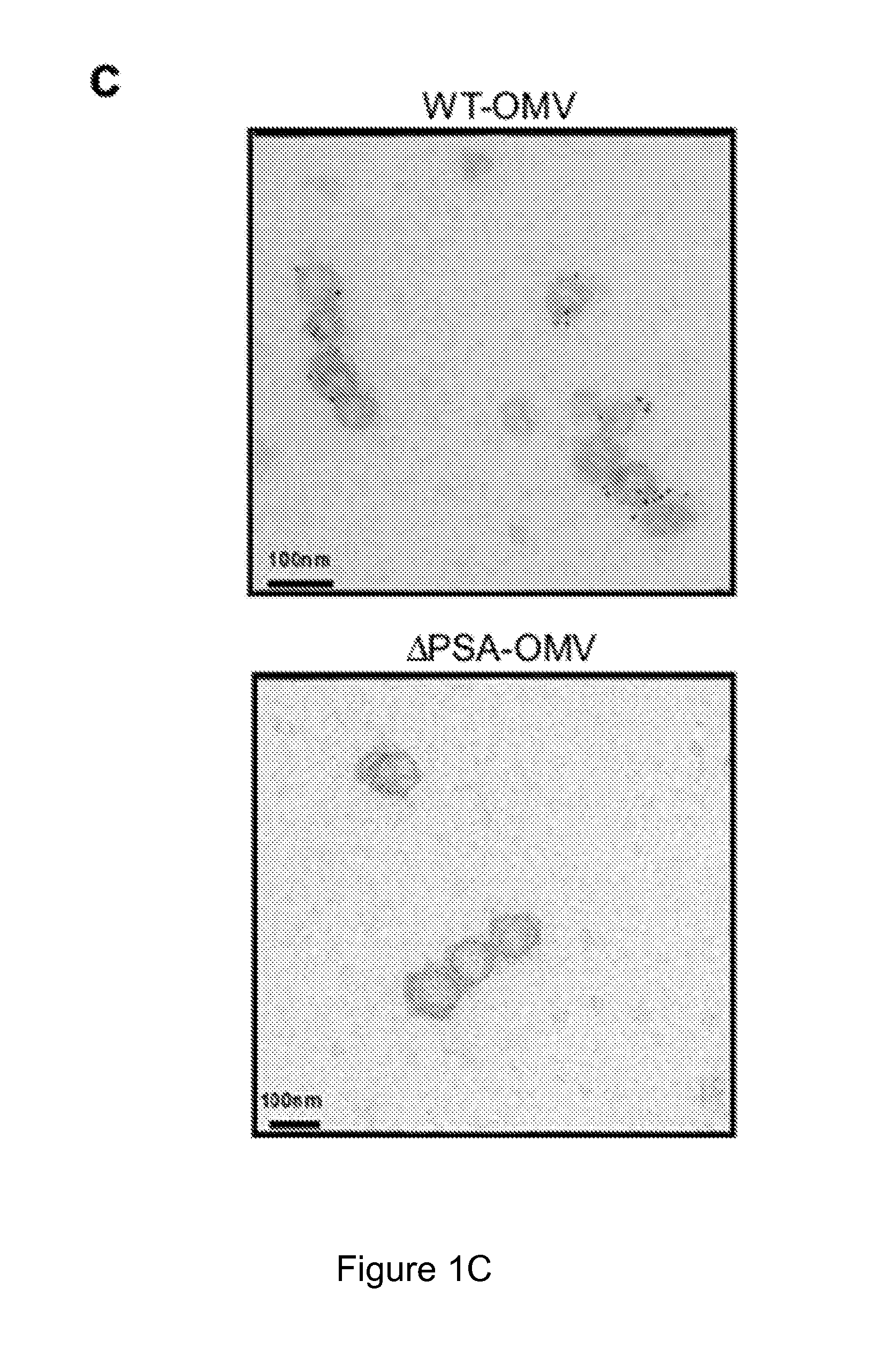
[0177]The results illustrated in FIG. 1a show that OMVs were abundantly produced by bacteria, and could be observed budding from the bacterial envelope (FIG. 1a, higher magnification). Applicants' previous studies have shown that deletion of PSA abrogates the immunomodulatory capacity of B. fragilis (Mazmanian, Liu, Tzianabos, and Kasper (2005) An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 122:1 107-118) (Mazmanian, Round, and Kasper (2008) A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 453 (7195) 620-625. Electron micrographs of a PSA mutant strain (B. fragilis ΔPSA) illustrate no defect in OMV synthesis, and the size, shape and abundance of OMVs produced wer...
example 2
OMVs Protect Animals from Experimental Colitis and Intestinal Inflammation in a PSA-Dependent Manner
[0184]To investigate if OMVs can ameliorate clinical symptoms of disease, mice were treated by gavage with OMVs during the induction of TNBS (2,4,6-trinitrobenzene sulfonic acid) colitis.
[0185]The results illustrated in FIG. 2a indicate that control animals rapidly lost weight following rectal administration of TNBS (FIG. 2a; TNBS+PBS), which did not recover compared to vehicle treated mice (FIG. 2a; ETOH+PBS). Remarkably, OMVs given orally to TNBS animals significantly protected from weight loss (FIG. 2a; TNBS+WT-OMV). Most importantly, when OMVs from B. fragilis ΔPSA were administered, weight loss was indistinguishable from TNBS animals (FIG. 2a; TNBS+ΔPSA-OMV), demonstrating that PSA is responsible for preventing wasting disease.
[0186]Our efforts to detect intact vesicles in the colon after intra-gastric gavage were confounded by observations of host-derived vesicles, even in germ-...
example 3
PSA Containing OMVs Inhibits TNF-α / IL-17, Enhances IL-10 Expression
[0192]The production of canonical pro- and anti-inflammatory cytokines associated with colitis was measured in mice treated as exemplified in Example 2. In particular, cytokine transcript analysis was performed by qRT-PCR from RNA recovered from whole colons or purified CD4+ T cell from mesenteric lymph nodes.
[0193]The relevant results representative of 3 independent trials are reported in FIG. 2e (whole colons) and FIG. 2f (purified CD4+ T cell from mesenteric lymph nodes). Those results show that transcript levels of pro-inflammatory biomarkers / cytokines tumor necrosis factor (TNF-α) and IL-17A were elevated in TNBS− treated animals, but reduced by OMV administration in a PSA-dependent manner (FIG. 2e). Consistent with protection from pathology, OMVs elicited the production of increased anti-inflammatory biomarkers / cytokines IL-10 levels compared to animals orally given ΔPSA-OMV (FIG. 2f). Analysis of cytokine prod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com