Application of 5-aminolevulinic acid in preparation of product for preventing and treating inflammatory bowel disease
A technology of aminolevulinic acid and inflammatory bowel disease, which is applied in the field of application of 5-aminolevulinic acid in the preparation of products for the prevention and treatment of inflammatory bowel disease, and can solve the problems that have not yet been related to 5-aminolevulinic acid. To achieve the effect of alleviating inflammatory bowel disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
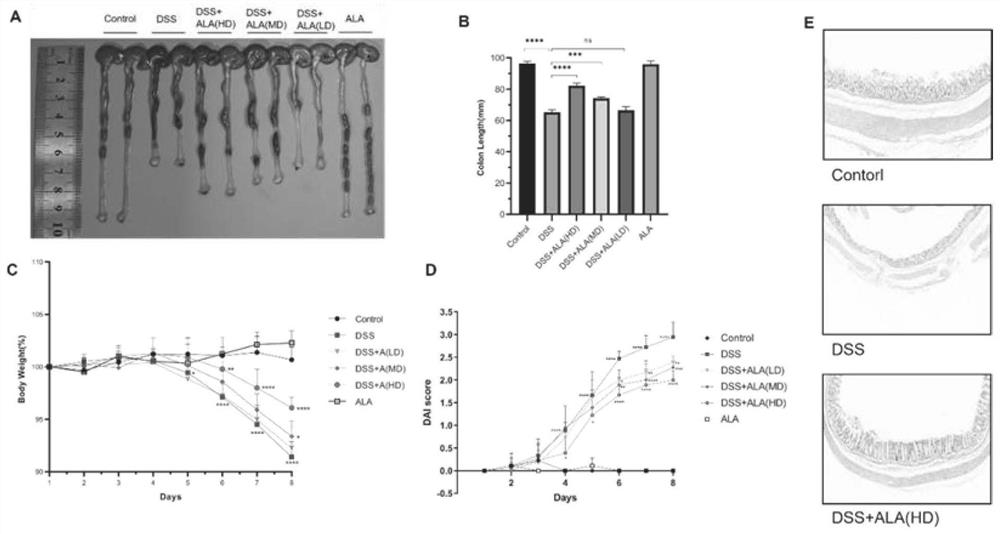
[0026] Six-week-old C57BL / 6 mice were used to establish an experimental colitis model with DSS. Intervention by intragastric administration of 5-ALA, once a day for 7 days, divided into three dose groups, among which the high dose group (HD) was 200mg / kg, the middle dose group (MD) was 100mg / kg, and the low dose group (LD) was 50mg / kg. The mice that were not treated with DSS were used as the blank control group (control group), and the colitis model mice that were not given 5-ALA intragastric intervention were used as the negative control group (DSS group). The colon length, body weight, disease, and intestinal mucosal changes of the five groups of mice were counted, and the results were as follows: figure 1 shown.
[0027] Depend on figure 1 It can be seen that after the intervention of 5-ALA intragastric administration in the middle dose group and the high dose group, the degree of shortening of the mouse colon was alleviated (pfigure 1 A-B), symptoms such as weight loss...
Embodiment 2
[0029] Intestinal tissues of mice in the control group, DSS group and 5-ALA high-dose intervention group in Example 1 were extracted for RNA-seq sequencing research. The results are shown in Table 1. Compared with mice with experimental colitis, the most significantly different Hub genes in the intestinal tissue of mice with experimental colitis administered 5-ALA include: Stat1, Irf7, Ifit3, Oasl2, Usp18, Rsad2, Isg15, Rtp4, Psmb8, Gbp2, these differential genes are important factors in the interferon-γ signaling pathway, which were all increased in intestinal tissue of mice with enteritis, but decreased after 5-ALA intervention.
[0030] Table 1 RNA-seq sequencing results
[0031] gene name LogFC value of 5-ALA intervention group v.s.DSS group Stat1 -1.891525777 Irf7 -3.122429087 Ifit3 -3.529132031 Oasl2 -3.650766538 Usp18 -2.411050953 Rsad2 -1.836606601 Isg15 -4.20883179 Rtp4 -2.46127824 Psmb8 -1.85042157 ...
Embodiment 3
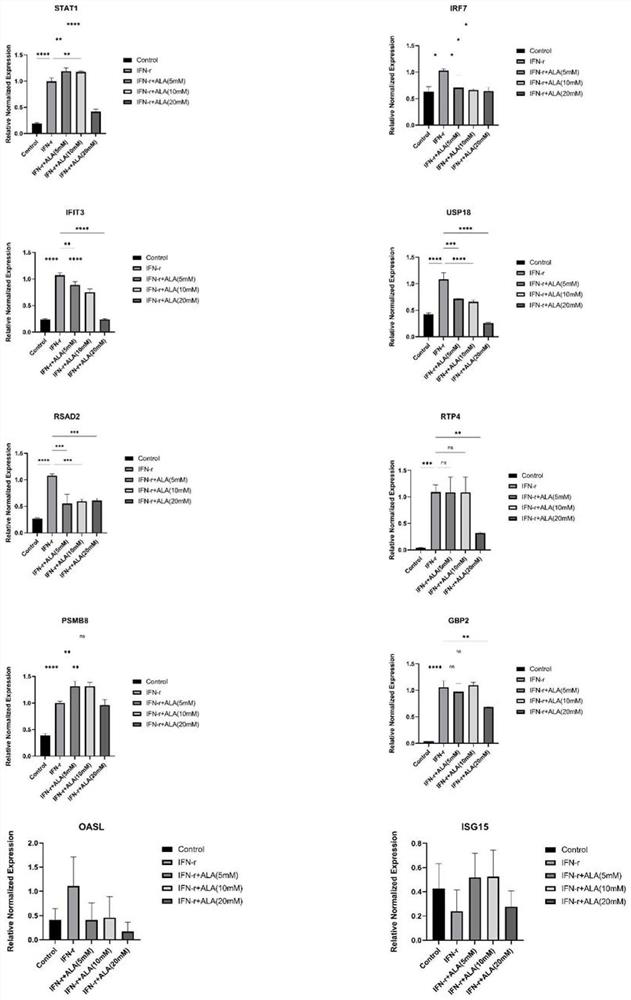
[0033] Human normal intestinal epithelial cells NCM46024h were intervened with human recombinant IFN-γ at a final concentration of 20ng / mL to create a cell inflammation model, and at the same time intervened with 5-ALA, divided into 5 groups: Control, IFN-γ, IFN-γ+ALA (5mM ), IFN-γ+ALA (10mM), IFN-γ+ALA (20mM). The expression levels of Stat1, Irf7, Ifit3, Oasl2, Usp18, Rsad2, Isg15, Rtp4, Psmb8, and Gbp2 genes were detected in each group, and the results were as follows: figure 2 shown.
[0034] Depend on figure 2 It can be seen that after the intervention of 5-ALA with a final concentration of 20mM, Stat1, Irf7, Ifit3, Oasl2, Usp18, Rsad2, Isg15, Rtp4, Psmb8, Gbp2 and other genes were significantly decreased, proving that 5-ALA intervention can reduce the interferon pathway levels of related genes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com