Anti-inflammatory and mydriatic intracameral solutions for inhibition of postoperative ocular inflammatory conditions
a technology of ocular inflammatory conditions and intracameral solutions, which is applied in the direction of sense disorder, organic active ingredients, drug compositions, etc., can solve the problems of increased risk of injuring eye structures, increased risk of eye injury, so as to promote mydriasis, and inhibit a postoperative inflammatory condition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Clinical Studies Evaluating Phenylephrine 1% / Ketorolac 0.3% in Cataract Surgery and Intraocular Lens Replacement for Maintenance of Mydriasis and Prevention of Postoperative Pain
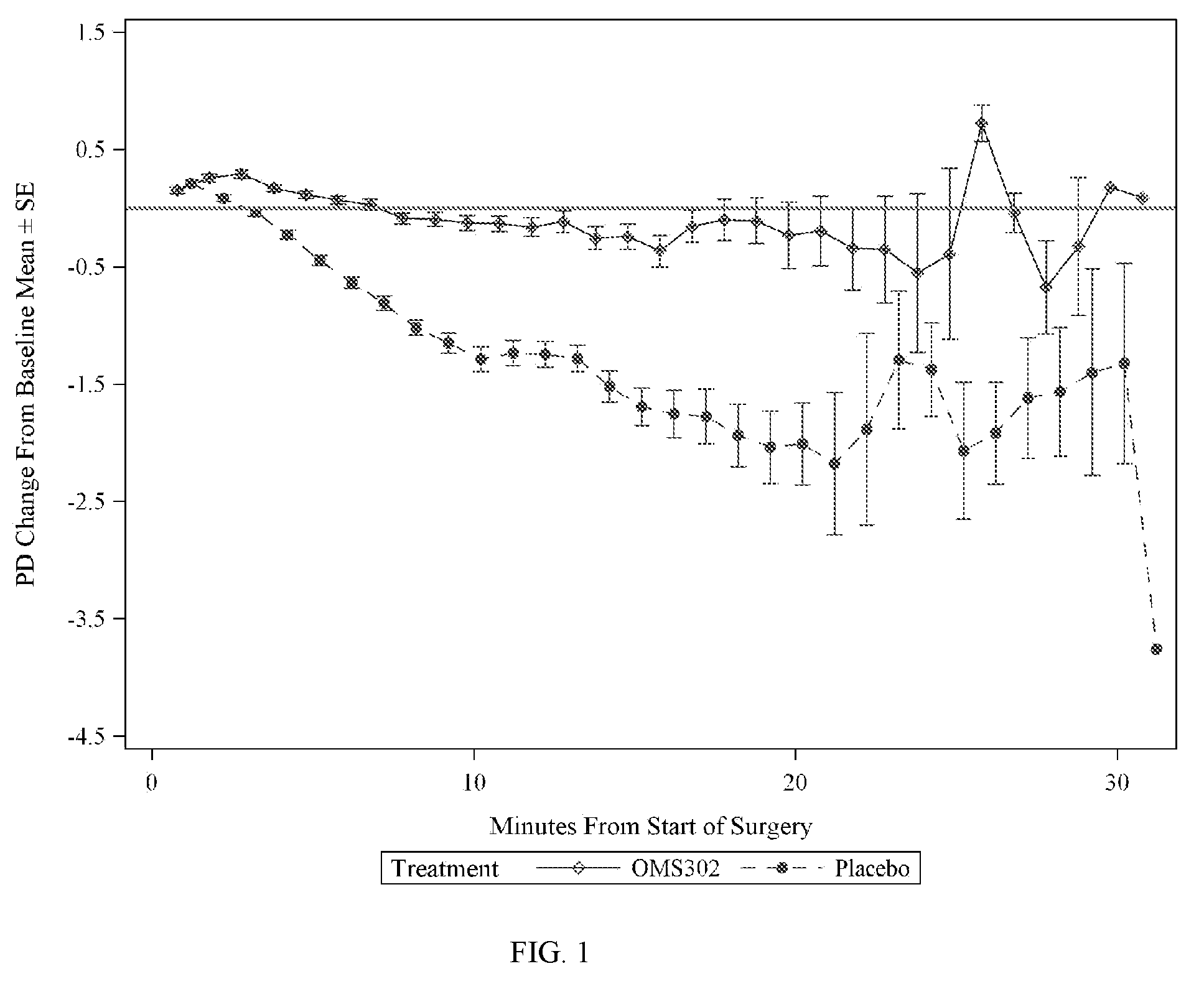
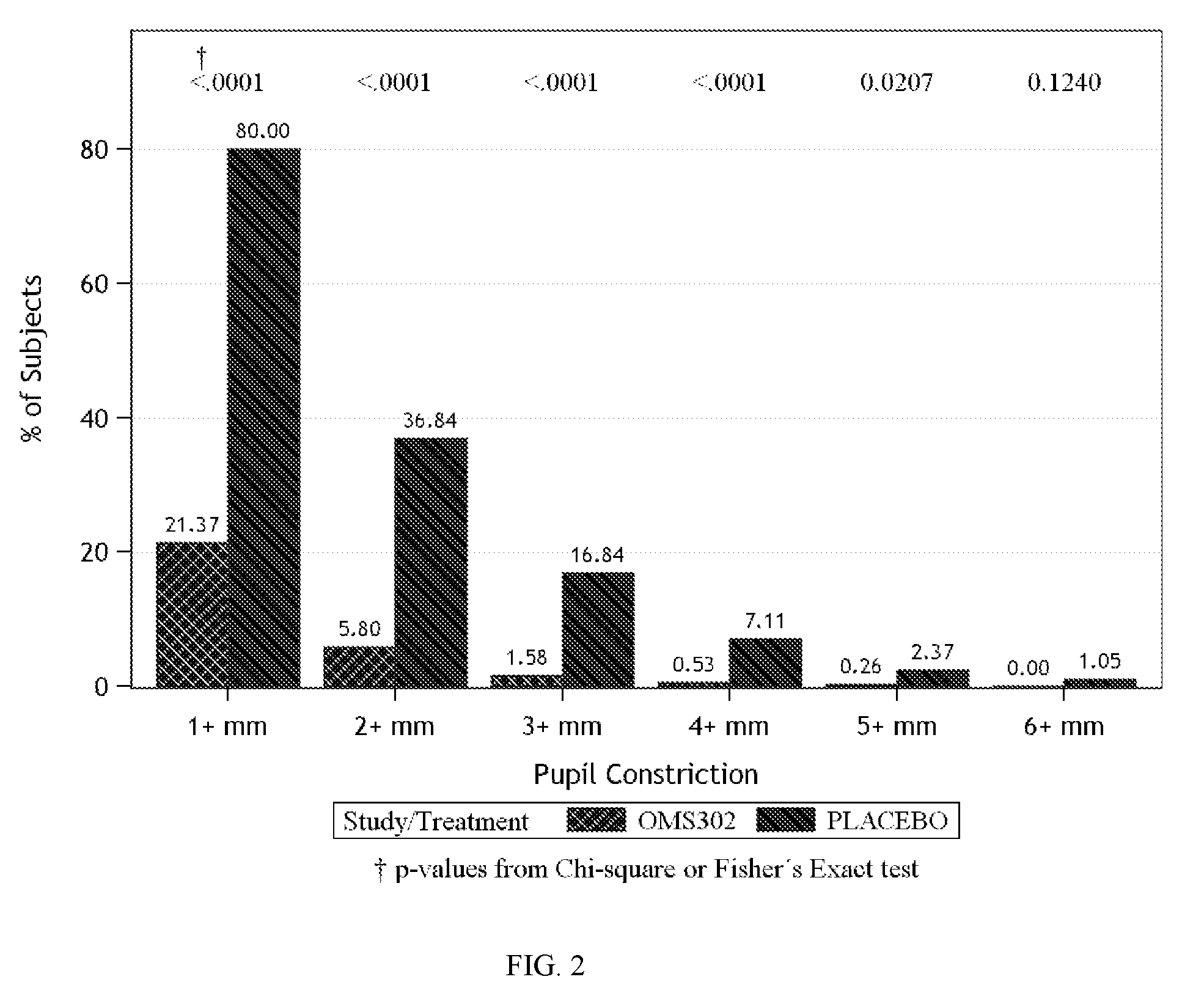
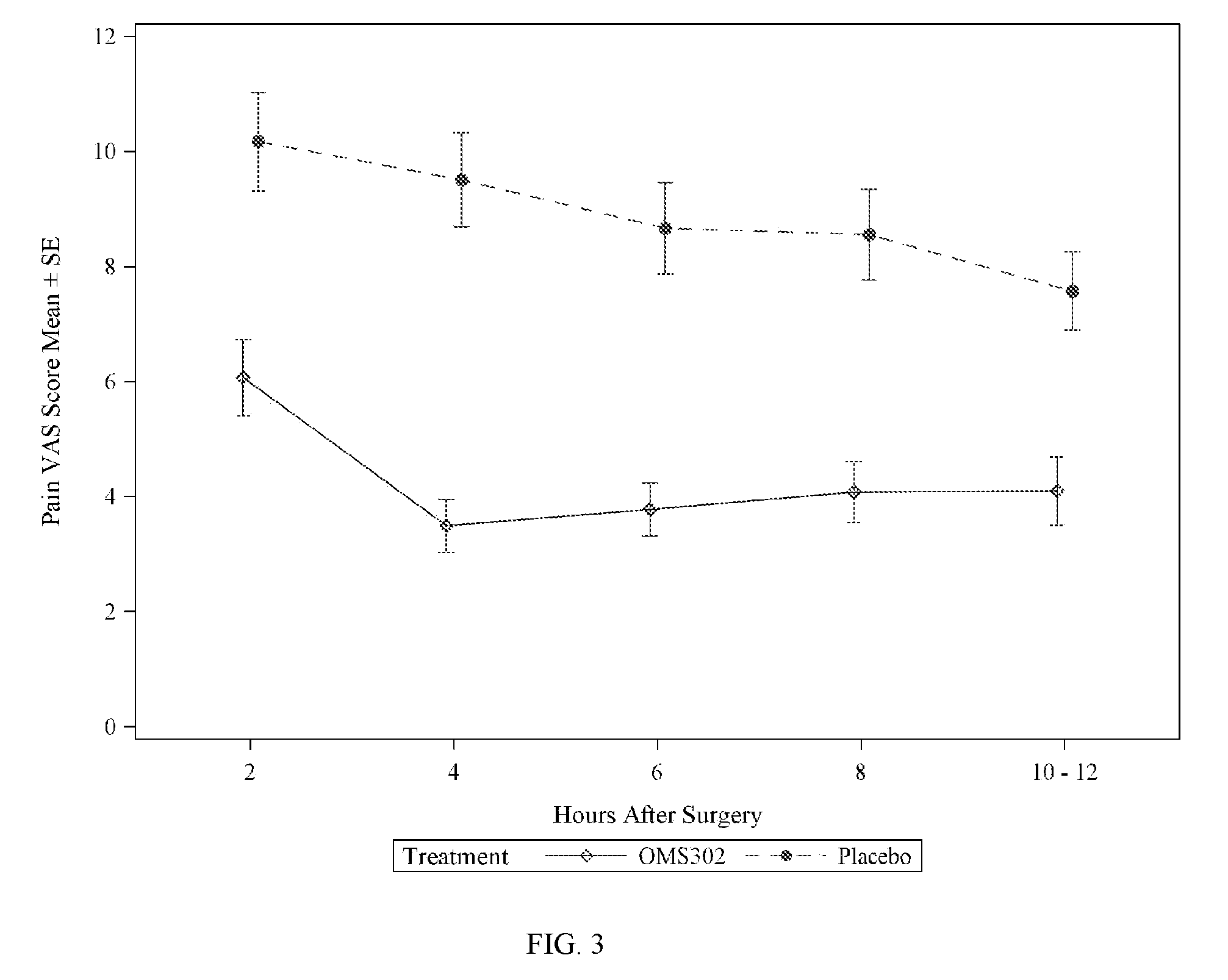
[0077]This example describes two Phase 3 clinical studies performed to evaluate the efficacy and safety of phenylephrine 1% and ketorolac 0.3% injection formulated as described in Table 1 above when used for the maintenance of mydriasis during, and prevention of postoperative pain following, cataract surgery and intraocular lens (IOL) replacement.
[0078]Methods
[0079]Two pivotal, multi-center, randomized, parallel-group, double-masked, placebo-controlled phase 3 studies (Study 1 and Study 2) that were conducted to support the use of phenylephrine 1% and ketorolac 0.3% injection (OMS302) for maintaining intraoperative mydriasis, preventing intraoperative miosis, and reducing early postoperative ocular pain associated with cataract surgery and IOL replacement. A total of 20 sites in the United States and Netherl...
example 2
Ocular Tissue Distribution of Ketorolac Following Administration of Phenylephrine 1% / Ketorolac 0.3% to Dogs During Intraocular Lens Replacement
[0098]This example describes the results of an in vivo study in dogs to determine the concentrations of ketorolac in the retina and other ocular tissues following the intracameral administration of phenylephrine 1% and ketorolac 0.3% injection formulated as described in Table 1 (OMS302) during IOL replacement in dogs.
[0099]Methods
[0100]IOL replacement by phacoemulsification was performed on 20 female beagles. During the procedure, OMS302 was administered in BSS solution via irrigation and intracameral injection immediately post-procedure. The target dose level of ketorolac was 5.71 mg / eye, and the target dose volume of OMS302 diluted in BSS solution was 250 mL per eye. Four animals per time point were sacrificed at 0, 2, 6, 8, and 10 hours post-procedure. Samples of blood and aqueous humor were collected. Enucleated eyes were frozen and disse...
example 3
Clinical Study Evaluating Intracameral Ketorolac Concentration Following Topical Ketorolac Administration Prior to Cataract Surgery
[0105]This example describes the results of a clinical study to determine postoperative intracameral concentrations of ketorolac in subjects receiving topical ketorolac prior to cataract surgery.
[0106]Methods
[0107]Patients undergoing cataract extraction and lens replacement (CELR) were eligible. Written informed consent was obtained from 14 subjects, each of whom received topical ophthalmic ketorolac according to the surgeon's usual practice, beginning one day preoperatively. Immediately prior to the initial surgical incision, the surgeon withdrew a 100-μL sample of aqueous humor from the operative eye with a 30-gauge tuberculin syringe. At the conclusion of CELR prior to final re-inflation of the anterior chamber and wound closure, the surgeon withdrew another 100-μL sample from the anterior chamber. The ketorolac concentrations of the intracameral flui...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com