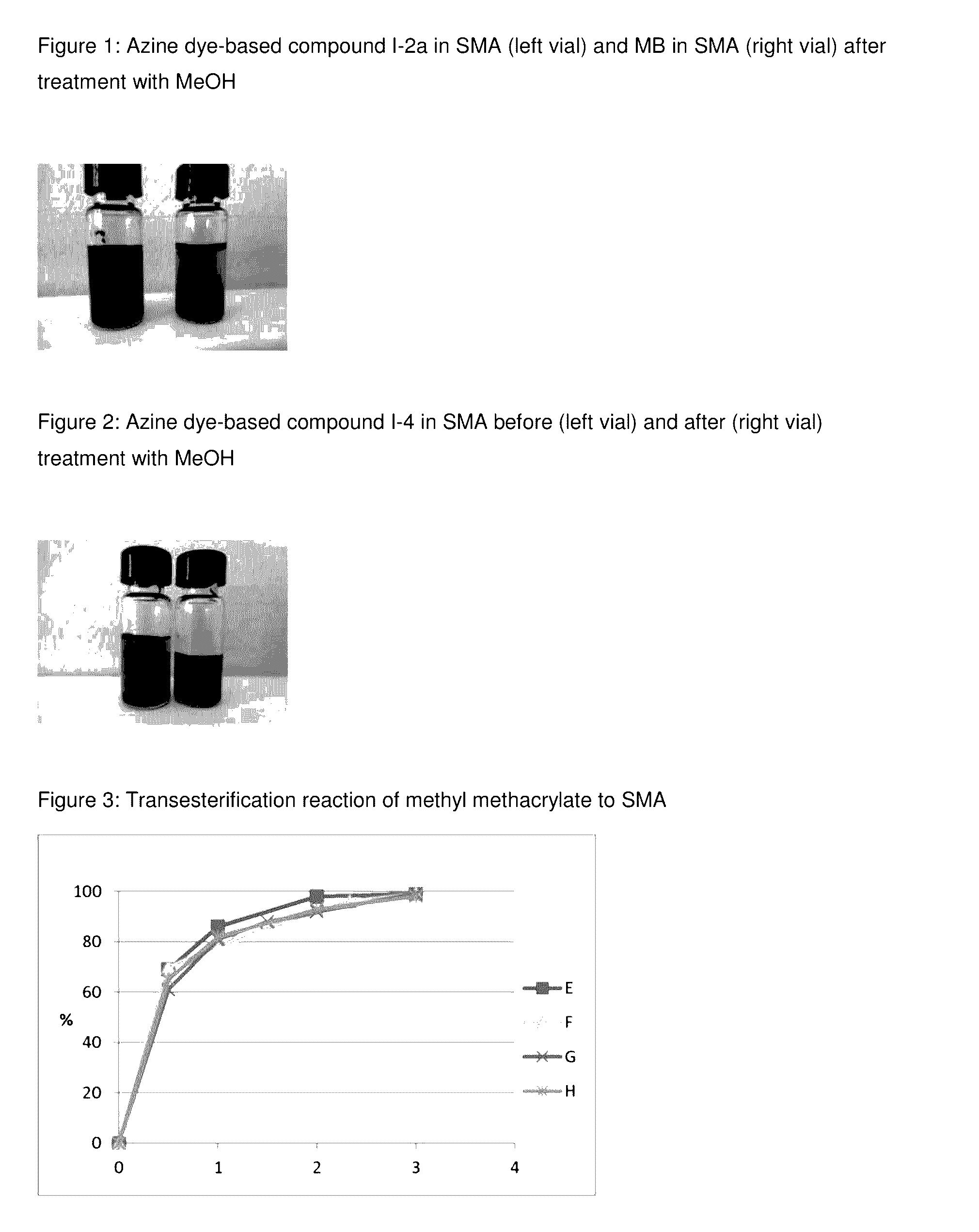
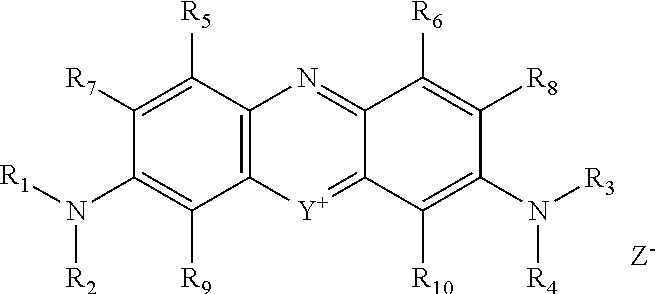
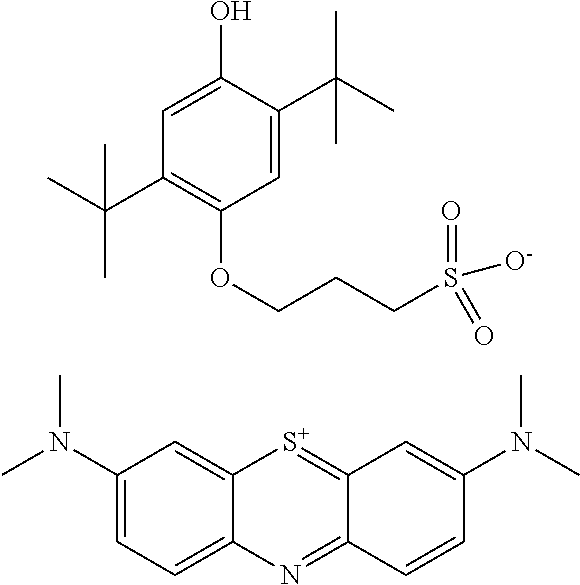
Method for prevention of premature polymerization
a polymerization and prevention technology, applied in the field of prevention of premature polymerization, can solve the problems of reducing the overall process of stabilizing the polymerizable compound against premature polymerization, so as to achieve the effect of preventing premature polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0084]The compound I-1 is prepared by heating 1 g (3.13 mmol) of methylene blue chloride and 0.675 g (3.13 mmol) of sodium 1-octanesulfonate at reflux for 24 h in 100 mL of CH2Cl2 and 10 mL of water. The layers are separated, and the organic phase is washed with water (3×10 mL) and dried over magnesium sulfate. Filtration and concentration gave 1.49 g (75%) of the octanesulfonate salt of methylene blue.
example 2
[0085]The compounds I-1a to I-3a are prepared in situ (by mixing the starting components with a polymerizable compound, see below). As pre-treatment a 1:1 molar mixture of the constituent salts was finely ground. This is done to homogenize the mixture and to provide intimate contact between both salts.
example 3
[0086]The compound I-4 is prepared by deprotonation of 1 g of 2,5-di-tert-butylhydroquinone (DBHQ) (4.50 mmol) in the presence of 0.13 g sodium hydride (5.40 mmol) in 20 mL THF at room temperature. The reaction mixture was stirred at room temperature for 15 minutes, then 0.55 g of 1,3-propanesultone (4.50 mmol) is added slowly and the reaction mixture is stirred overnight. The excess sodium hydride is quenched by slow addition of methanol. Precipitation with Et2O followed by filtration and washing with Et2O yields 1.57 g of a white solid (95%). Anion exchange with methylene blue is performed as described above via extraction from water with dichloromethane.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com