Method and system for determining integrated metabolic baseline and potential of living cells
a technology of living cells and baselines, applied in the field of living cell baselines and potentials, can solve the problems of prior approaches that are limited to stimulating and analyzing prior approaches are not able to identify and deliver compounds capable of stimulating both major energetic pathways simultaneously, and prior approaches are not able to measure the simultaneous response of the two major energetic pathways to stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
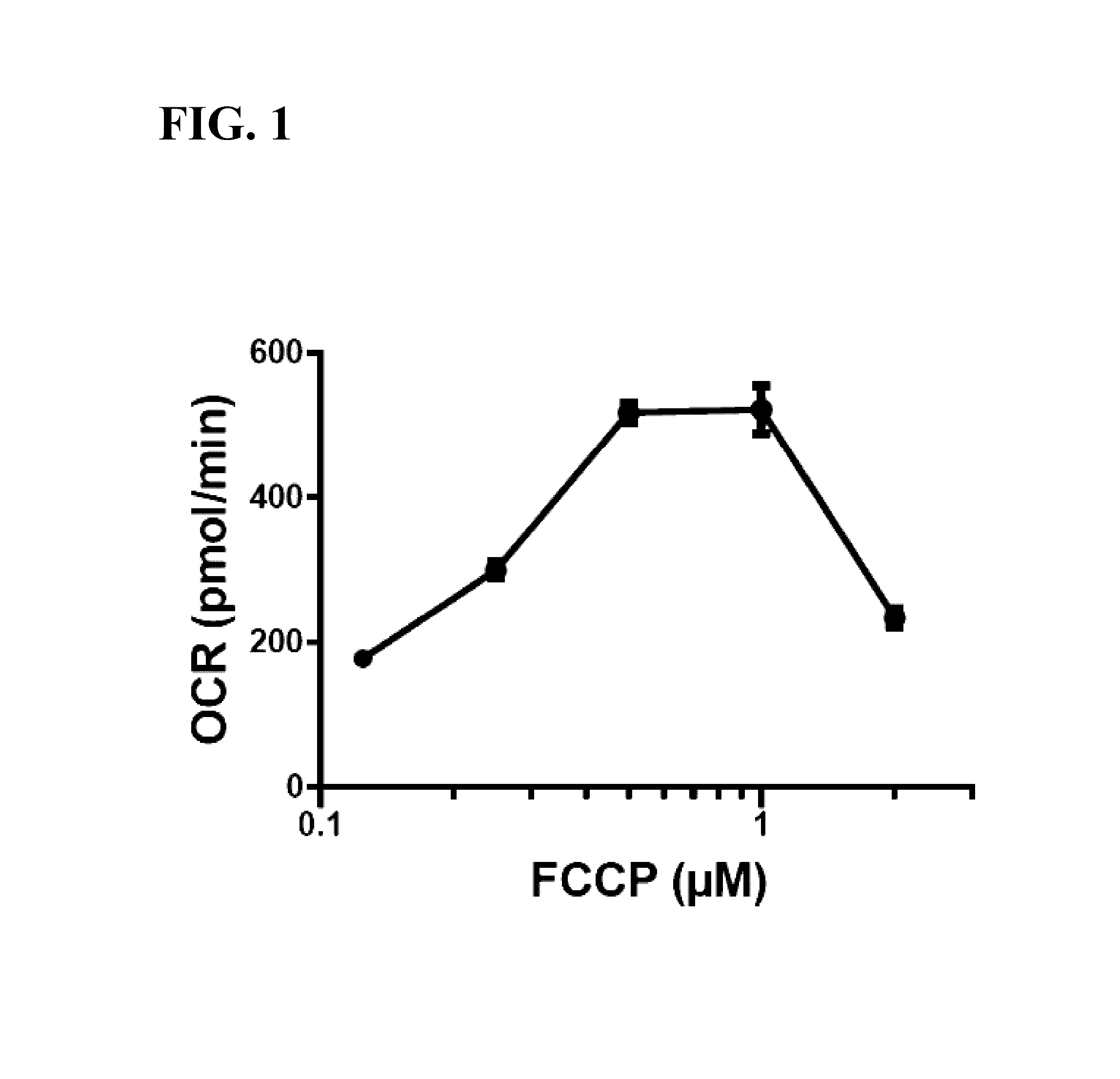
FCCP Titration in the Presence of Oligomycin in RAW 264.7 Macrophage Cells
[0064]RAW 264.7 macrophage cells were seeded into XFe96 microplates (Seahorse Bioscience) at a density of 8.0×104 cells / well. Because RAW 264.7 cells are semi-adherent, cells were added to each well and the microplate was then centrifuged at 300×g for two minutes to settle the cells to the bottom of the well. Cells were cultured for 24 hours in DMEM supplemented with 25 mM glucose, 4 mM glutamine, 1 mM pyruvate, and 10% FBS.
[0065]Culture media was replaced with assay media (modified DMEM omitting sodium bicarbonate; available from Seahorse Bioscience as XF Base Medium, supplemented with 10 mM glucose, 2 mM glutamine, and 1 mM pyruvate).
[0066]FCCP titration was performed using an XFe96 Extracellular Flux Analyzer (Seahorse Bioscience) and XFe96 assay cartridges (Seahorse Bioscience) according to the manufacturer's instructions. Briefly, cells were first treated with 1.0 μM oligomycin (final concentration in 200...
example 2
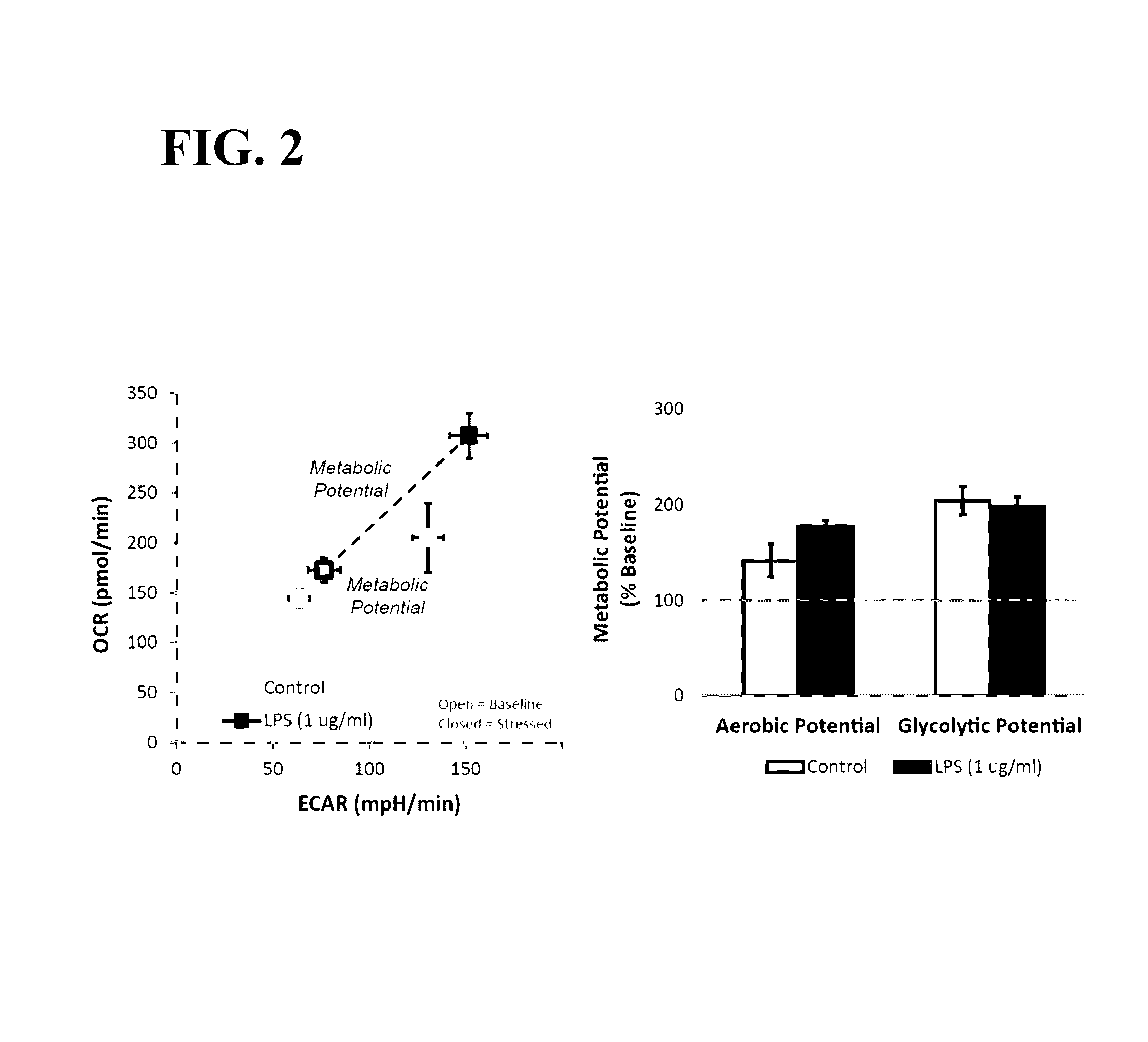
Effects of LPS Treatment on Metabolic Potential in RAW 264.7 Macrophage Cells
[0068]RAW 264.7 macrophage cells were seeded into XFp microplates at a density of 8.0×104 cells / well. Because RAW 264.7 cells are semi-adherent, cells were added to each well and the microplate was then centrifuged at 300×g for two minutes to settle the cells to the bottom of the well. Cells were cultured for 24 hours in DMEM supplemented with 25 mM glucose, 4 mM glutamine, 1 mM pyruvate, and 10% FBS.
[0069]Culture media was replaced with assay media (modified DMEM omitting sodium bicarbonate; available from Seahorse Bioscience as XF Base Medium, supplemented with 10 mM glucose, 2 mM glutamine, and 1 mM pyruvate). Cells treated with LPS received media containing 1 μg / ml LPS, control cells received assay media. Cells were incubated for 1 hour at 37° C. in a non-CO2 incubator before assay.
[0070]Metabolic potential was measured using an XFp Extracellular Flux Analyzer (Seahorse Bioscience) and XFp assay cartrid...
example 3
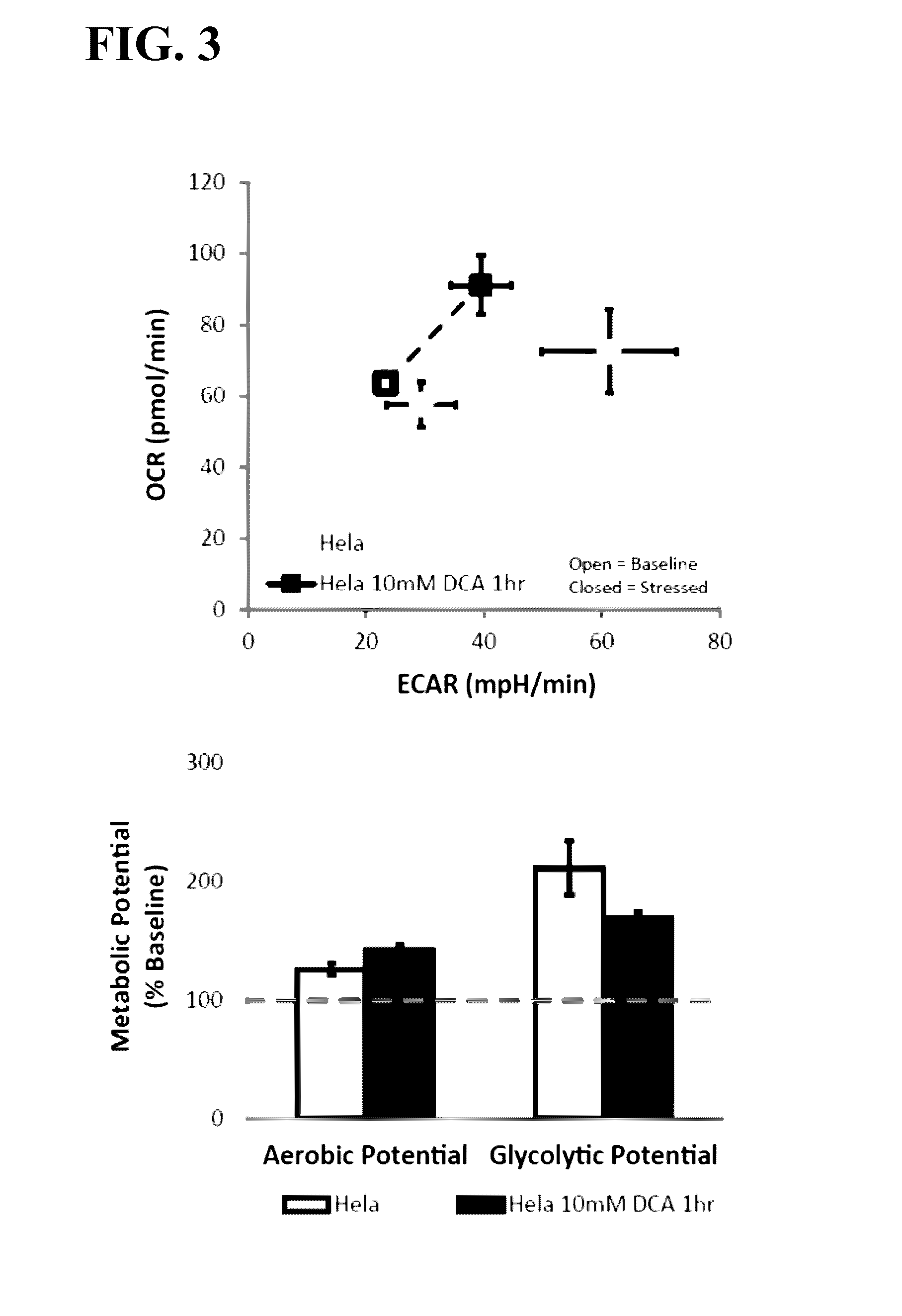
Effects of Treatment with DCA on Metabolic Potential of Hela Cells
[0072]Hela cells were seeded into XFp microplates at a density of 1.2×104 cells / well. Cells were cultured for 24 hours in MEM supplemented with 5.5 mM glucose, 2 mM glutamine, and 10% FBS.
[0073]Culture media was replaced with assay media (modified DMEM omitting sodium bicarbonate; available from Seahorse Bioscience as XF Base Medium, supplemented with 10 mM glucose, 2 mM glutamine, and 1 mM pyruvate). Treated cells received media containing 10 mM dichloroacetate (DCA), control cells received assay media. Cells were incubated for 1 hour at 37° C. in a non-CO2 incubator before assay.
[0074]Metabolic potential was measured using an XFp Extracellular Flux Analyzer (Seahorse Bioscience) and XFp assay cartridges (Seahorse Bioscience). Briefly, microplates containing DCA-treated and control cells were placed in the XFp Extracellular Flux Analyzer, and initial OCR and ECAR measurements are taken. Treated and control wells are ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com