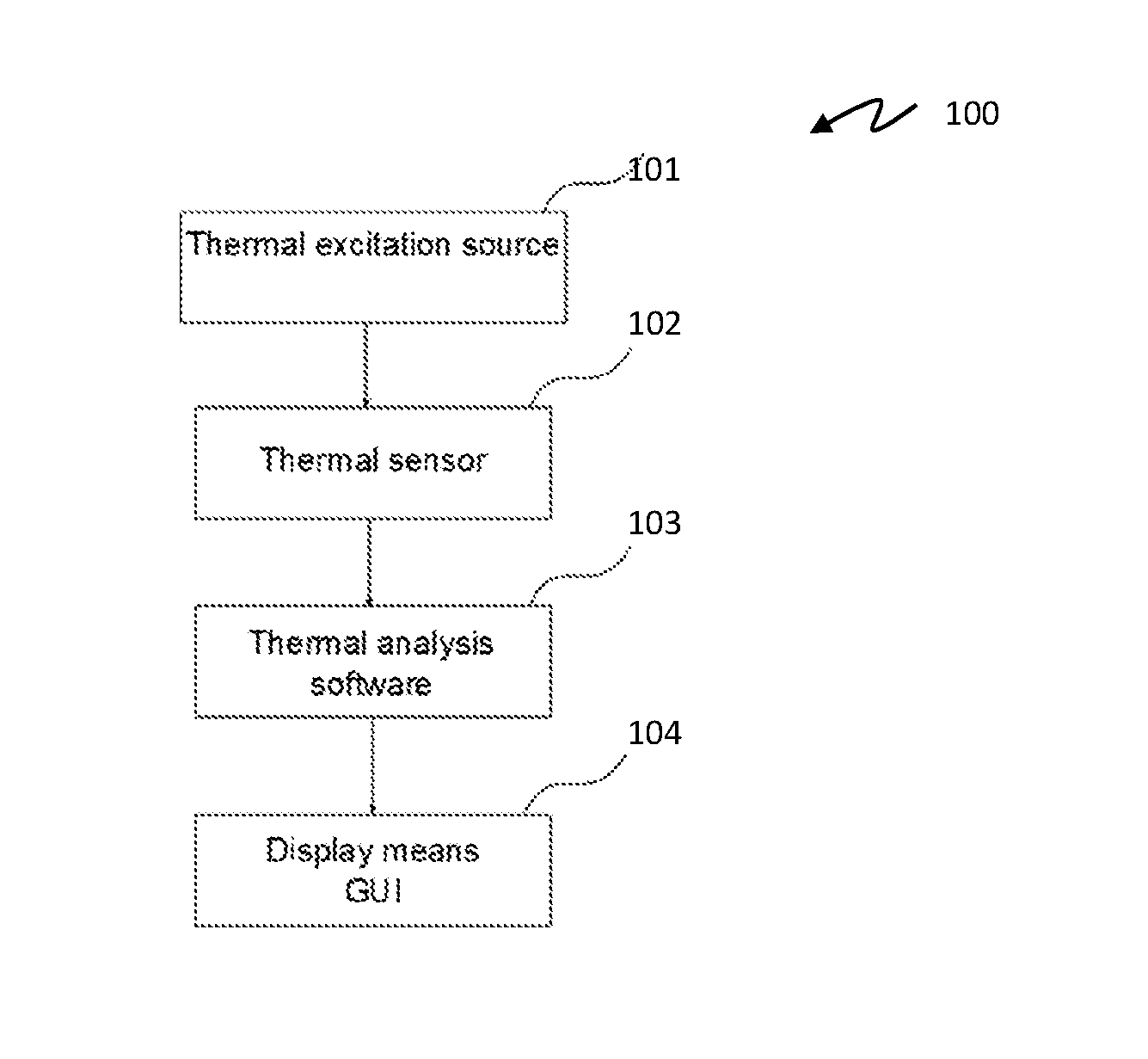
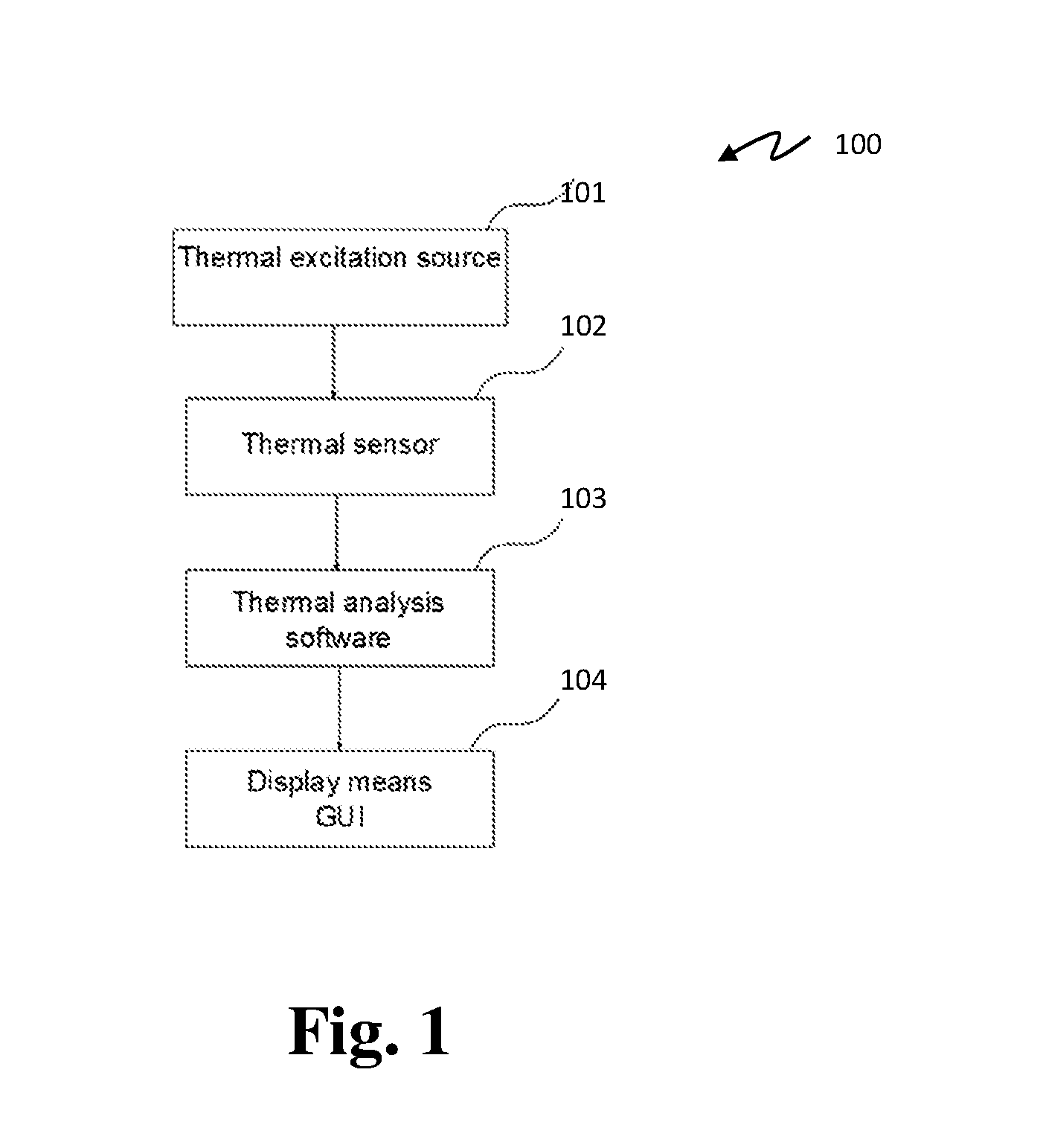
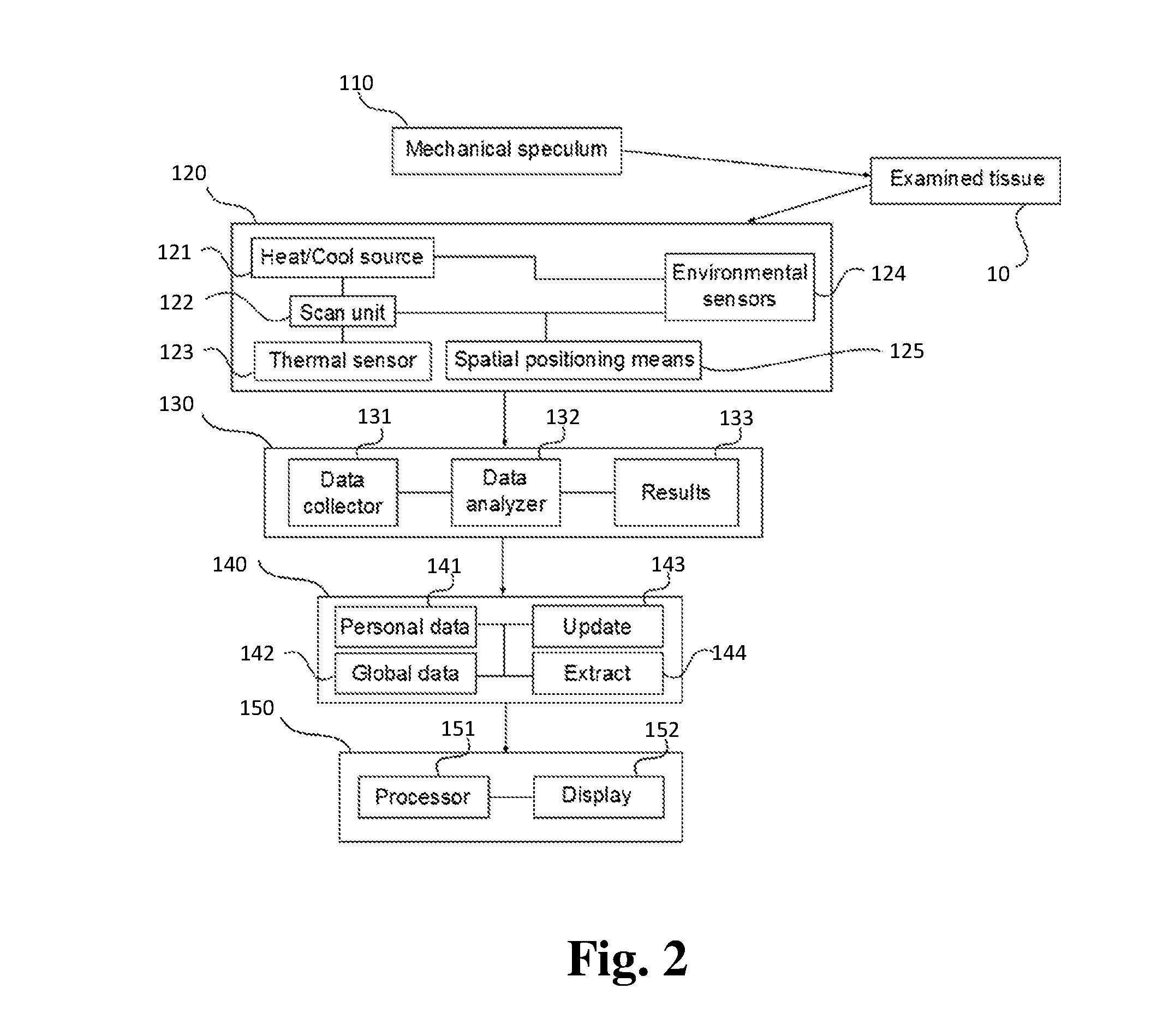
A device and method for cancer detection, diagnosis and treatment guidance using active thermal imaging
a technology of active thermal imaging and cancer detection, applied in the field of cancer detection and diagnosis, can solve the problems of low survival rate of lung cancer, untreatable, and large tumor size, and achieve the effect of reducing the risk of cancer, reducing and improving the survival rate of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0307]The experimental set up used to evaluate the invention is comprised of two stages. The second stage is designed to achieve greater accuracy and elaboration of the results obtained in the first stage, in addition to handling experimental issues and difficulties arising in the first experimental stage. The second stage was conducted in view of the results obtained in the first. Experimental design goes as follows:
[0308]Image capturing of all cell cultures; Laboratory conditions take into account: (a) Neutralizing disturbances; (b) Constant temperature, registration of any alterations. (c) Registration of humidity values.
[0309]Camera set up: Control set up—heating; Control set up—cooling; Conduct experiments using heating; Conduct experiments using cooling.
example 2
[0310]The system of the present invention can be configured as a ‘decision support’ system, informing a user such as a clinician as to whether a positive CT result is a cancer (true positive) and requires further investigation, or whether the positive CT result is a false positive.
[0311]With the system of the present invention, the results are immediate, do not involve radiation risks and are independent of an expert's eye. The test is computerized and automatic, with no need for a long, expensive analyzing stage.
[0312]As disclosed above, the technology is based upon analysis of the temperature decay profile and measurement of heat diffusion in the lung. It is well known that the density of cancerous cells is higher than that of normal cells, their shape is different and their nuclei are enlarged. These differences cause a fundamental change in the thermal properties of the cells. The planned operating principle—short heating followed by tracking diffusion of heat into the tissue an...
example 3
[0323]A simulation of a method of generating a thermal diffusion image is demonstrated on a horizontal slice of a human thorax (chest), which includes the lungs and the heart. FIG. 10 shows a CT image of a normal human thorax (1000), showing the heart (1010), spine (1020), lungs (1030), and the bones (1040), muscles (1050) and fat (1060). These have been overlaid with lines indicating the simplified shapes for the organs which will be used in the simulation. The spine (1020) is simulated by a triangle, the heart (1010) by an oval, with the inner and outer limits of the bone (1040), muscle (1050) and fat (1060) being indicated by concentric ovals. The lungs (1030) occupy the space between the oval of the heart (1010) and the triangle of the spine (1020) as inner limits to the lungs and the inner perimeter of the bone (1040) as the outer limits to the bone.
[0324]FIG. 11 shows the simplified shapes of the organs (1100), without the CT scan. In FIG. 11, the heart (1010) is indicated by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com