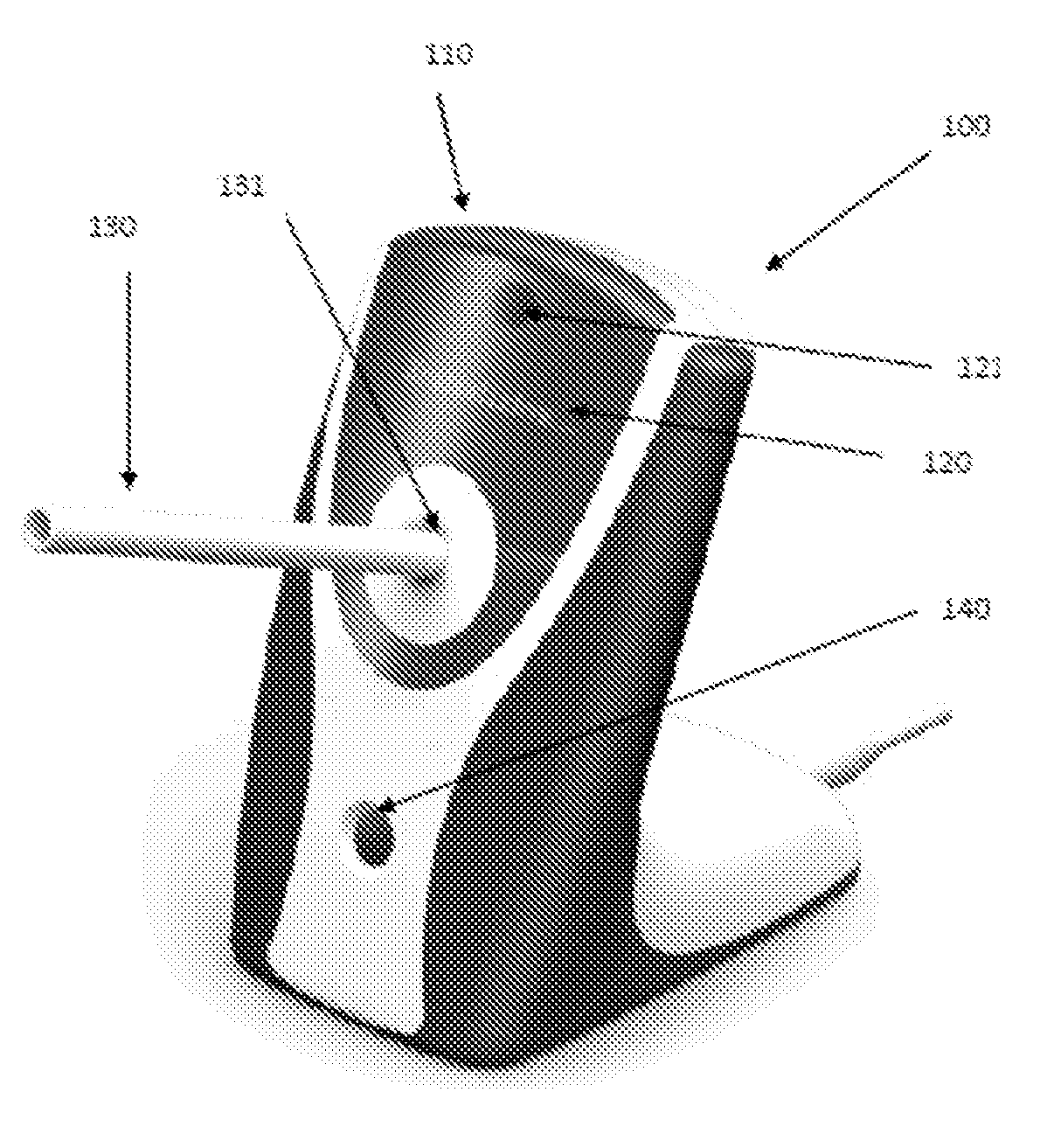
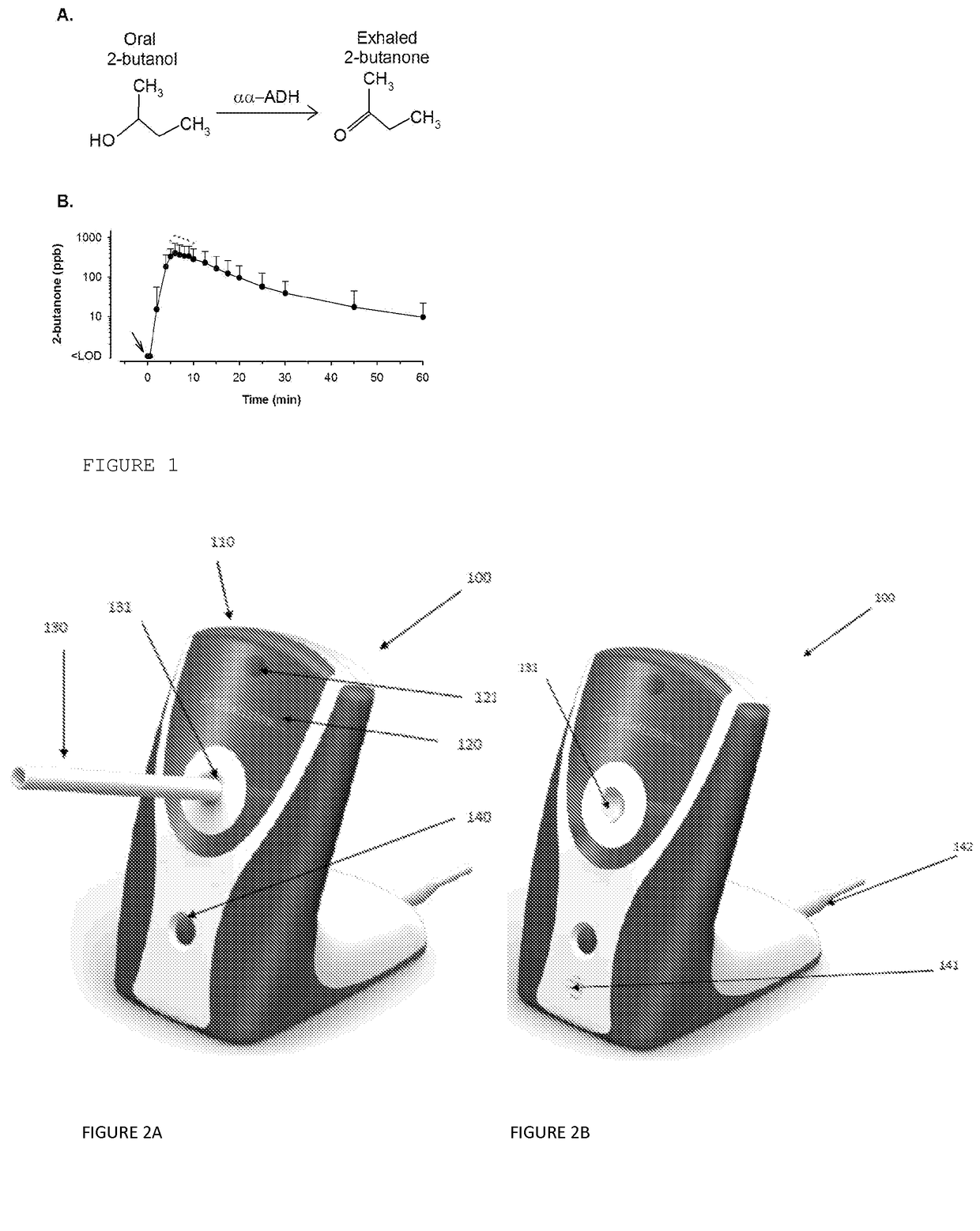
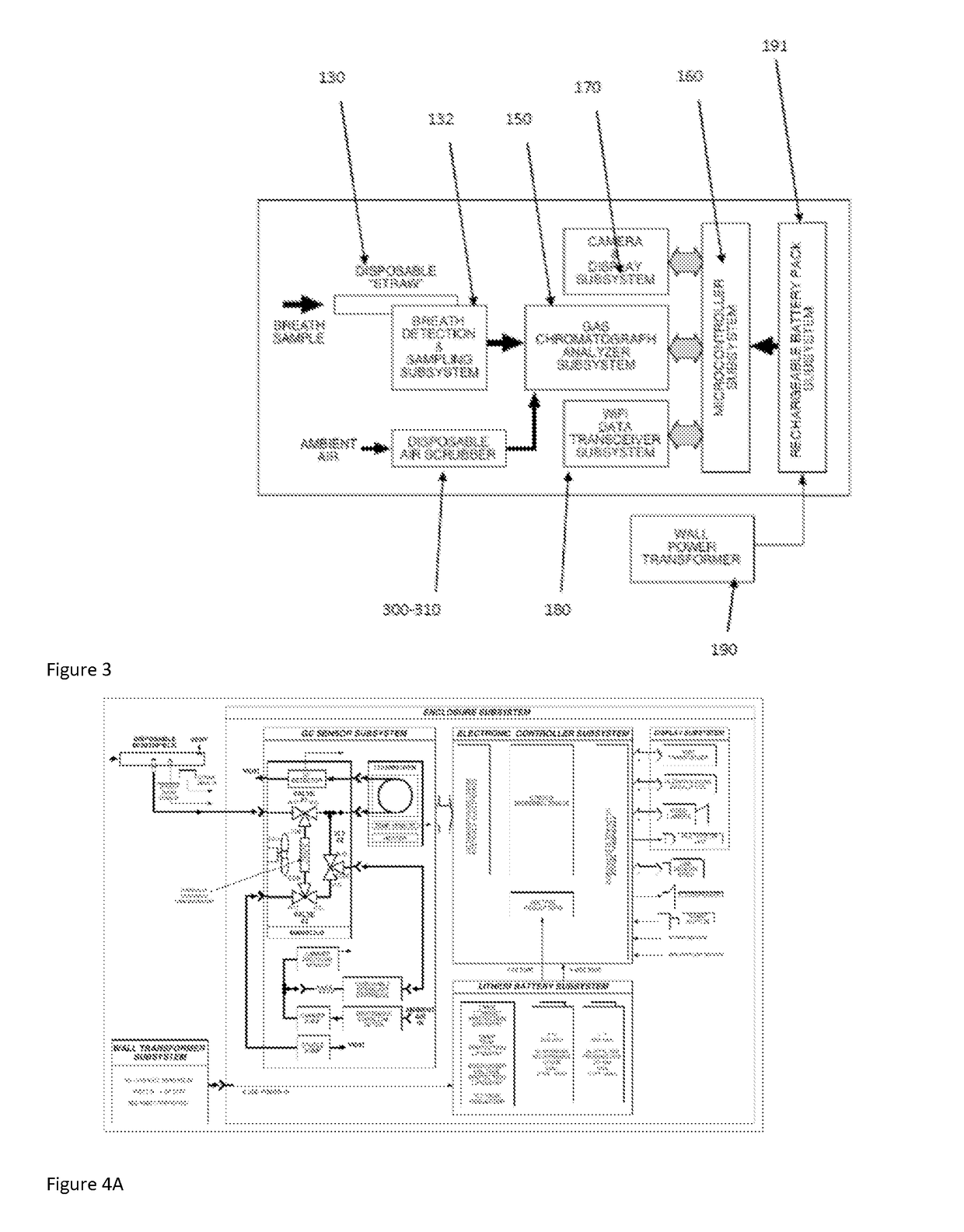
Medication adherence monitoring device
a technology of medication adherence and monitoring device, which is applied in the field of medication adherence monitoring system, can solve the problems of system insufficiency, methodological difficulty in assessing long-term adherent behavior with respect to medication regimen, and variability of non-adherence rate found, so as to facilitate rapid release of aem, facilitate stable storage of aem composition, and reliable filling of softgel capsules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Hardware Specifications and Performance—Type I Device
[0597]General Overview:
[0598]The SMART® mGC is capable of detecting aldehydes, ketones, esters, ethers, and miscellaneous volatile organic compounds with, e.g., boiling points between 20° C. (68° F.) and 98° C. (208° F.)
[0599]FIG. 9 shows a typical output chromatogram detecting key constituents in the breath, including acetone and isoprene, with clear separation of 2-butanone, derived from ingestion of 2-butanol.
[0600]In one specific embodiment of the present invention, the SMART® device has the following specifications. These specifications are provided to ensure a complete and enabling written description of this invention, but those skilled in the art will appreciate that these specifications should not be interpreted as limiting on the invention.[0601]Operating Principle: Isothermal gas chromatography using ambient air carrier gas and solid-state detector[0602]Enclosure Size: 4.1″×8.9″×2.1″ (3.6″ max)[0603]Weight: 2.5 lbs. (1....
example 2
SMART® mGC Chromatographic Separation of Acetone, Isoprene and Ethanol—Type I Device
[0646]As shown in FIG. 9, a very clean separation of ethanol, acetone and isoprene is achieved when these compounds are simultaneously adsorbed to the sample concentrator followed by thermal desorption, separation via the mGC, and detection by the MOS sensor.
example 3
Clinical (In Vivo, Human) and Potential Interferenent (In Vitro, Benchtop and Clinical) Studies to Optimize and Validate the SMART® System and Composition According to this Invention
[0647]To support development and facilitate regulatory filings, a number of complementary in vitro (benchtop: Interference Studies 1 through 4) and clinical (human: Clinical Studies 1 through 4) studies have been carried out to characterize the SMART® Adherence System. In terms of human exposure, the system has been safely used to date in 33 human studies (oral, sublingual, and microbicide administration routes), encompassing 1,318 experiments in 328 subjects and 8,524 breath analyses. Of particular note, three recent prospective, blinded, randomized, cross over clinical validation studies (127 subjects with 472 experiments and 2,464 breath analyses) using the SMART® Adherence System designed for oral medications were executed that focused on identifying an optimal adherence-enabling marker (AEM) formula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantitating volatile | aaaaa | aaaaa |
| volatile | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com