Peptide construct having a protease-cleavable linker
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
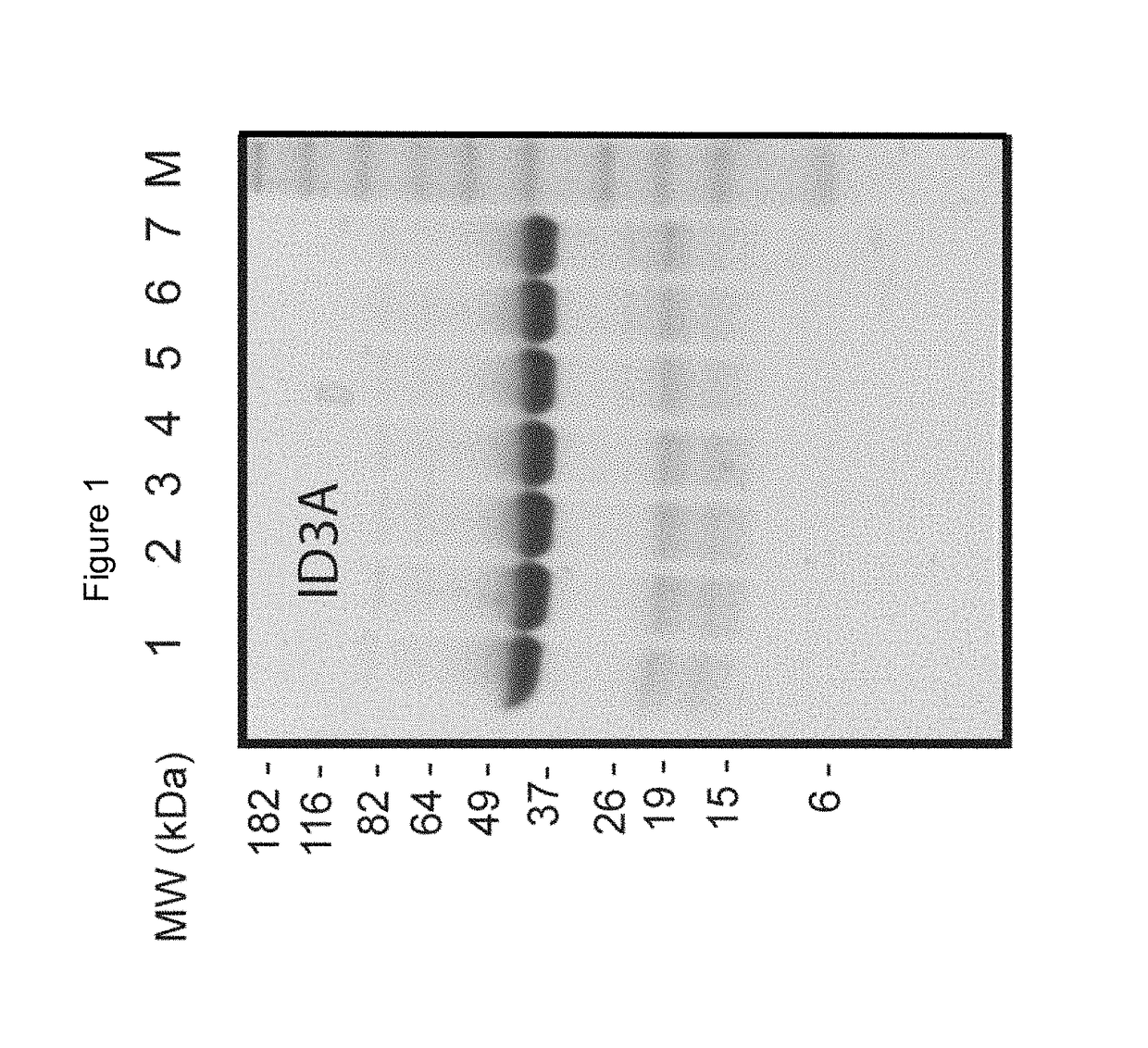
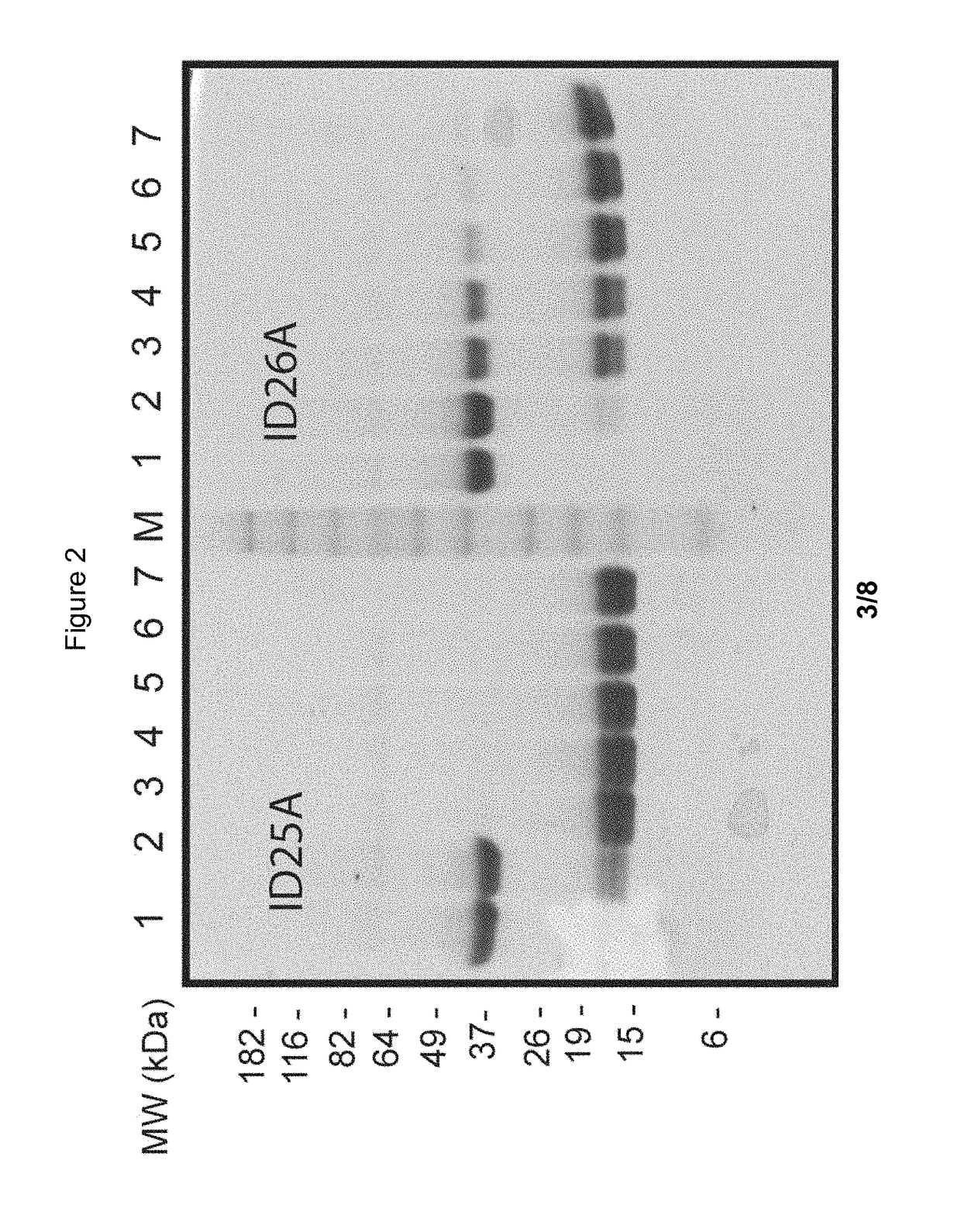
[0534]To test the lability of labile peptide linkers, the Trypsin Protease Assay was developed. This assay is performed as follows.
[0535]A buffered (10 mM acetic acid, pH 3.2, containing 0.01% thimerosal) aqueous suspension of L-1-tosylamido-2-phenylethyl chloromethyl ketone (TPCK)-treated Trypsin-agarose beads (trypsin from bovine pancreas; T4019; Sigma Aldrich) is used for the assay. The beads are washed 3 times with water (250 μl beads+1.25 ml water) followed by washing 5 times with Trypsin buffer (TRYP buffer; 1 mM Tris-HCl, 20 mM CaCl2 [pH 8.0]). Finally, the resin is resuspended in TRYP buffer as a 50% (v / v) suspension.
[0536]100 μl of a 2 mg / ml construct solution is mixed with 225 μl 50% (v / v) immobilized TPCK-treated Trypsin in TRYP buffer. After time intervals such as 0, 10, 15, 30, 45 and 60 minutes of incubation at 37° C. in a shaker, samples are taken as follows: resin is pelleted by a 1 min centrifugation step at 500×g, and a 40 μl sample is taken from t...
example 2
[0538]The Trypsin Protease Assay protocol can be used wherein Trypsin beads are substituted with Chymotrypsin beads.
example 3
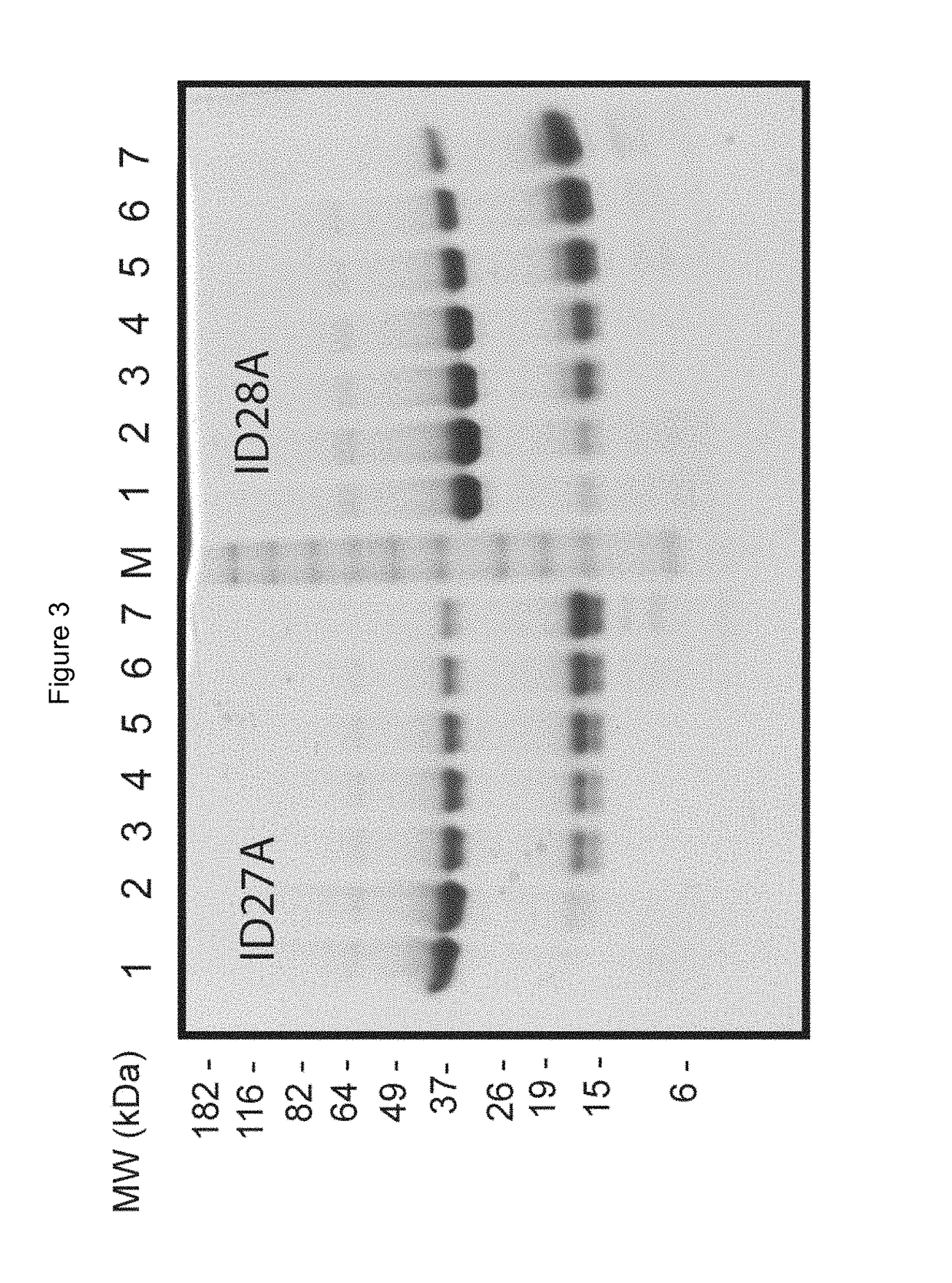
l Extract Protease Assay
[0539]To test the lability of labile peptide linkers, the Faecal Extract Protease Assay was developed. Faecal extract is a physiologically relevant matrix in particular for polypeptides targeted to the large intestine. This assay is performed as follows.
[0540]Human faecal samples from multiple individuals are turned into slurries with addition of 1×PBS at a ratio of 1, 2 or 3 mLs 1×PBS per gram of faeces. The slurries are then pooled (such that one pool represents the combined protease output from the faeces of multiple individuals), centrifuged and the supernatants removed, aliquoted and stored at −70 degrees C. This process removes the faecal matrix, including any cellular material. For digestion, constructs are incubated at a concentration of 160.16 μg / ml at 37° C. in pooled human faecal supernatant for 60 mins in the absence of BSA carrier. Aliquots are taken after time intervals such as 0, 10, 30 and 60 minutes and are mixed 1:1 in Protease Stop Solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com