Reduction of implant infection via tunable stimulation of localized adaptive immune response
a implant technology, applied in the field of implant infection reduction via tunable stimulation of localized adaptive immune response, can solve the problems of increasing health care costs, requiring repetitive hospital visits, and requiring removal or replacement of implanted devices or materials, so as to promote a regulated and localized immune response to bacteria, reduce or eliminate implant-related bacterial infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Degradable Micro- or Nano-Particles Cultured With Mouse Macrophage Cell Lines
[0112]Cell line: Mouse macrophage cell line RAW 264.7 was purchased from ATCC. The RAW 264.7 line was established from a tumor induced by Abelson murine leukemia virus. RAW 264.7 cells are negative for surface immunoglobulin, 1a, and Thy-1.2. These cells do not secrete detectable virus particles. RAW 264.7 cells were seeded onto 75 cm2 flasks at a subcultivation ratio of 1:3 to 1:6. Medium was replaced or added every 2 to 3 days. Cells were not passaged for more than 30 days.
[0113]Cell culture: RAW 264.7 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen). Subcultures were prepared by scraping. Cells were maintained at 95% air, 5% CO2 at a temperature of 37.0° C.
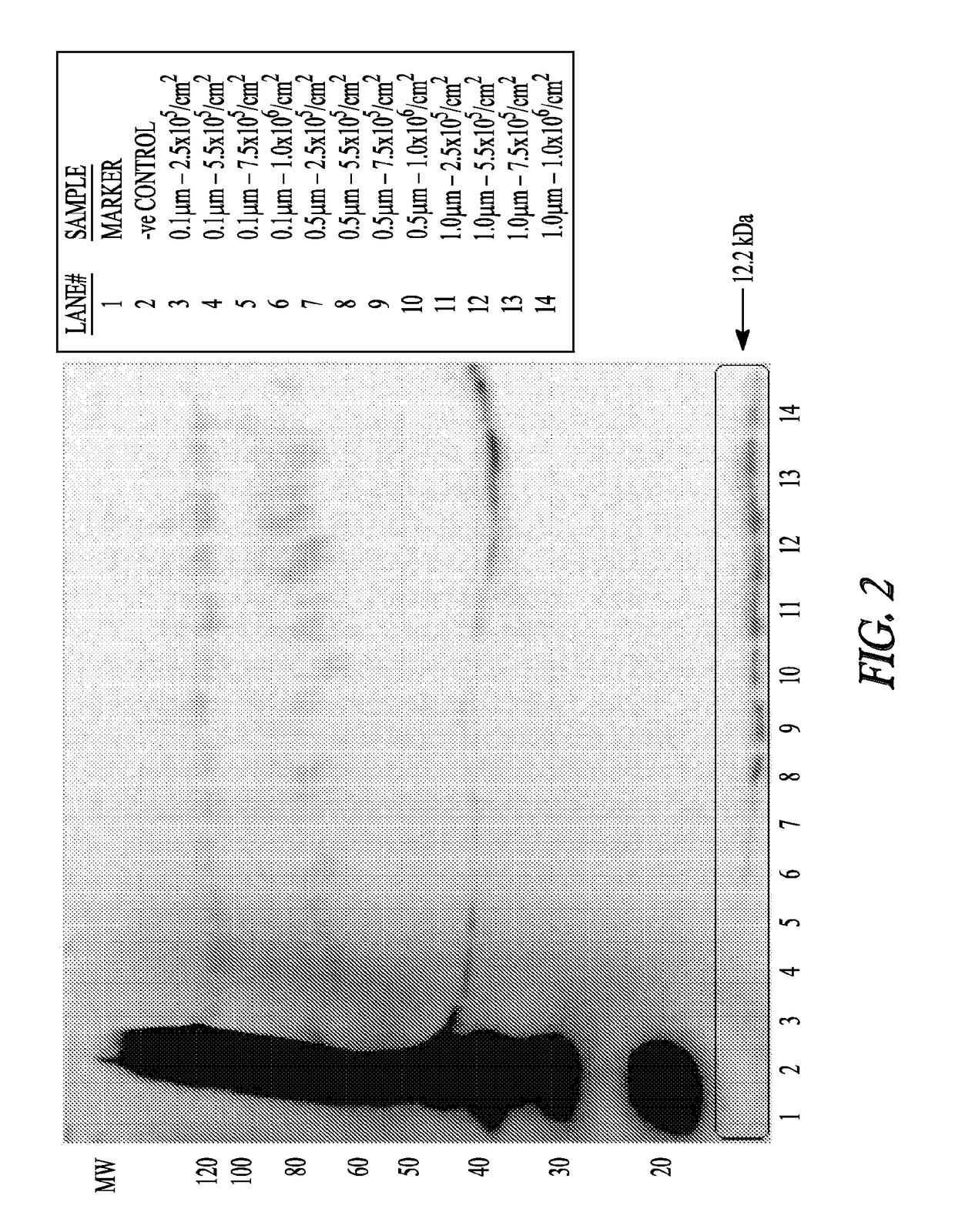
[0114]Micro- or nano-sphere incubations: Polystyrene micro-spheres or nanospheres (Corpuscular, Inc.) were tested for endotoxin levels and were confirmed to be 5, 5.5×105...
example 2
Degradable Micro- and Nano-Particles Trigger a Transitory, Tunable Response That is Localized to the Site of Injection in Animals
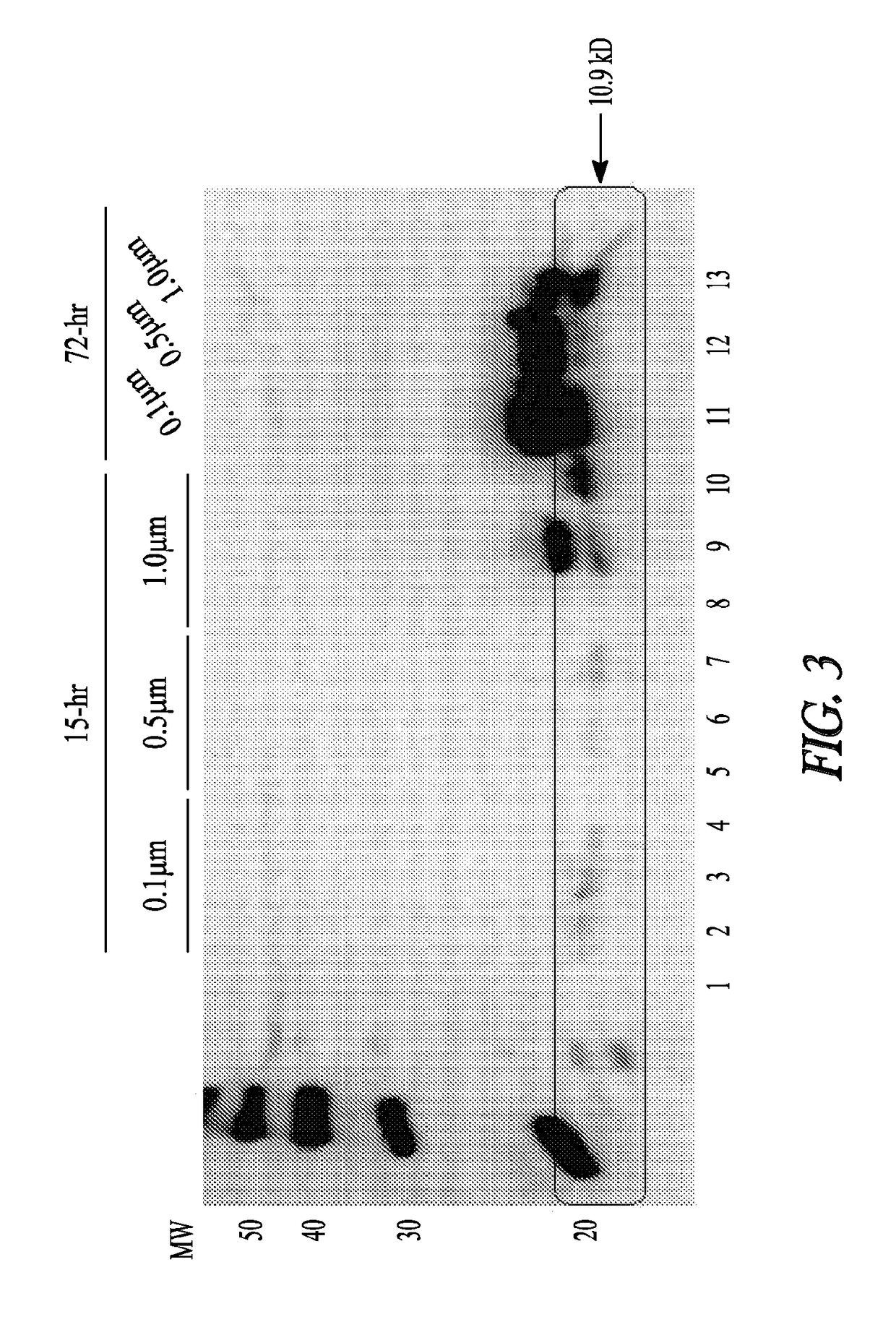
[0120]Animal Studies: 250-300 g Sprague Dawley rats were anesthetized with 75 mg / kg ketamine and 10 mg / kg xylazine. Fur was shaved above each quadriceps muscle. Particles were injected in 5 μl to 40 μl amounts in 5 locations in both muscles at a 1:100 or 1:50 dilution. Polystyrene microspheres (1.0 μM, 25 mg / ml) were first sterilized in 80% ethanol by three 50 min centrifugation steps followed by three washes in sterile PBS. The particles were then resuspended in sterile PBS at a concentration of 2.5 mg / ml. Prior to injection, the particles were further diluted 1:50 and 1:100 in sterile PBS. After 3 days, rats were anesthetized with 75 mg / kg ketamine and 10 mg / kg xylazine. The muscles were removed quickly, snap frozen into cold isopentane, placed in a tube, and further frozen in liquid nitrogen. Blood was collected from the heart, and the animal was euthan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com