Biomarker for cardiac disorders
a biomarker and cardiac disease technology, applied in the field of methods, assays and kits, can solve the problems of limited experimental evidence concerning direct translational action of uorfs upon, evidence of uorf-derived peptides, and the cost of increasing the number, so as to improve the sensitivity and false positive performance of cardiac troponin, and improve the sensitivity and false positive performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
nd Materials
Chemicals
[0190]Synthetic human TnTuORF, TnTuORF(Tyr), TnTuORF(Cys) and amino acids 1-7 of the signal peptide of vesicular integral membrane protein 36 (VIP36sp(1-7)) were synthesised by Mimotopes (Melbourne, Australia) at greater than 95% purity by mass spectrometry and high performace liquid chromatography (HPLC). All other synthetic or purified proteins were purchased from Hytest (Turku, FI), Peptide Institute (Osaka, Japan) or Sigma-Aldrich (St Louis, USA).
Human TnTuORF Assay Development
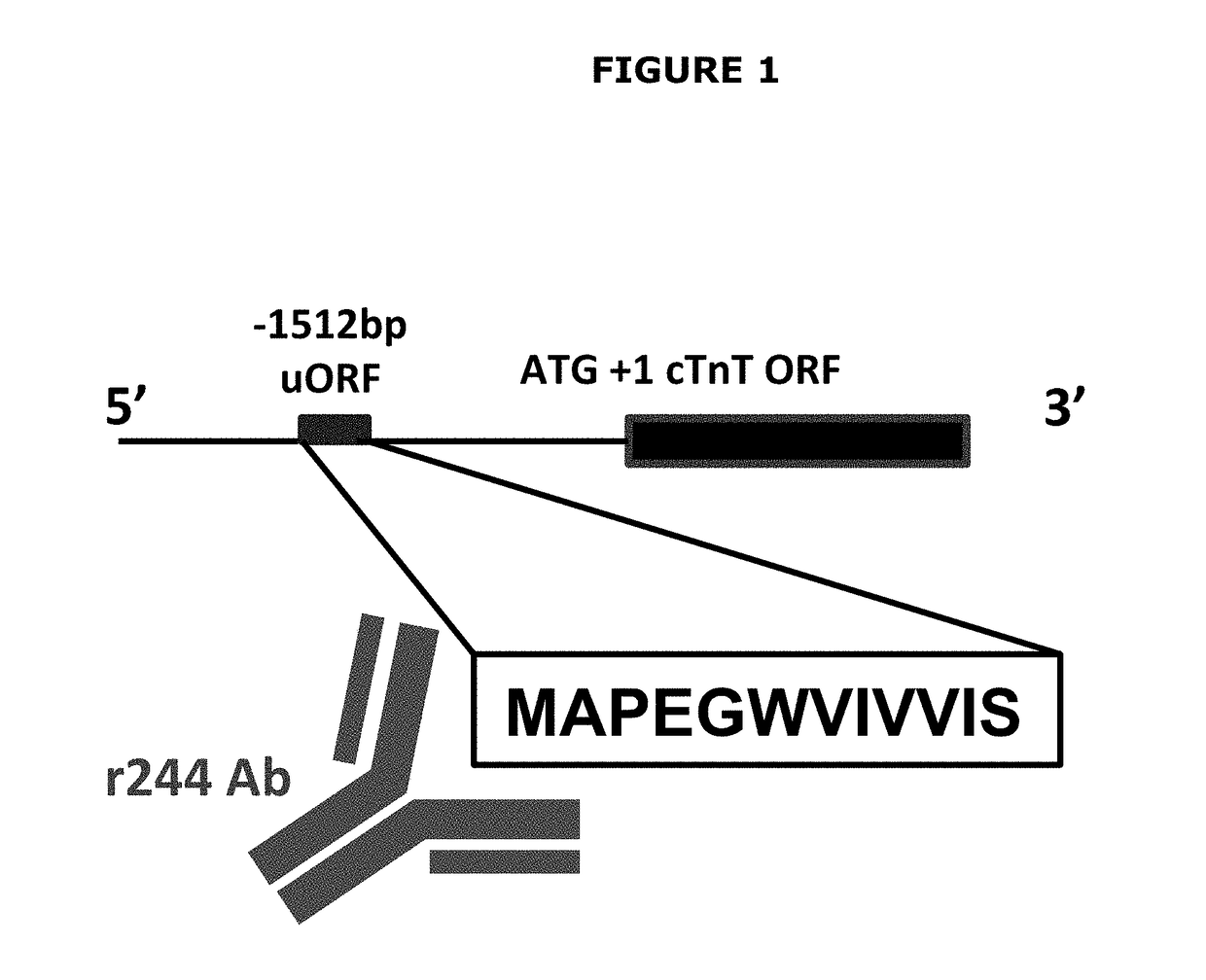
[0191]Assessment of the 5′ region upstream of the major open reading frame of human cardiac troponin T (NCBI Accession NG_007556) identified a putative 12 amino acid peptide (full amino acid sequence MAPEGWVIVVIS; SEQ ID NO:1) encoded 1,512 bp upstream of the major open reading frame (ORF) initiation site, with a Kozak score of +2 and a high likelihood of being efficiently translated (FIG. 1). This sequence was not seen in fast (NCBI Accession NG_013085) or slow (NCBI Accession NG_0118...
example 2
ation of Endogenous TnTuORF in Human Plasma
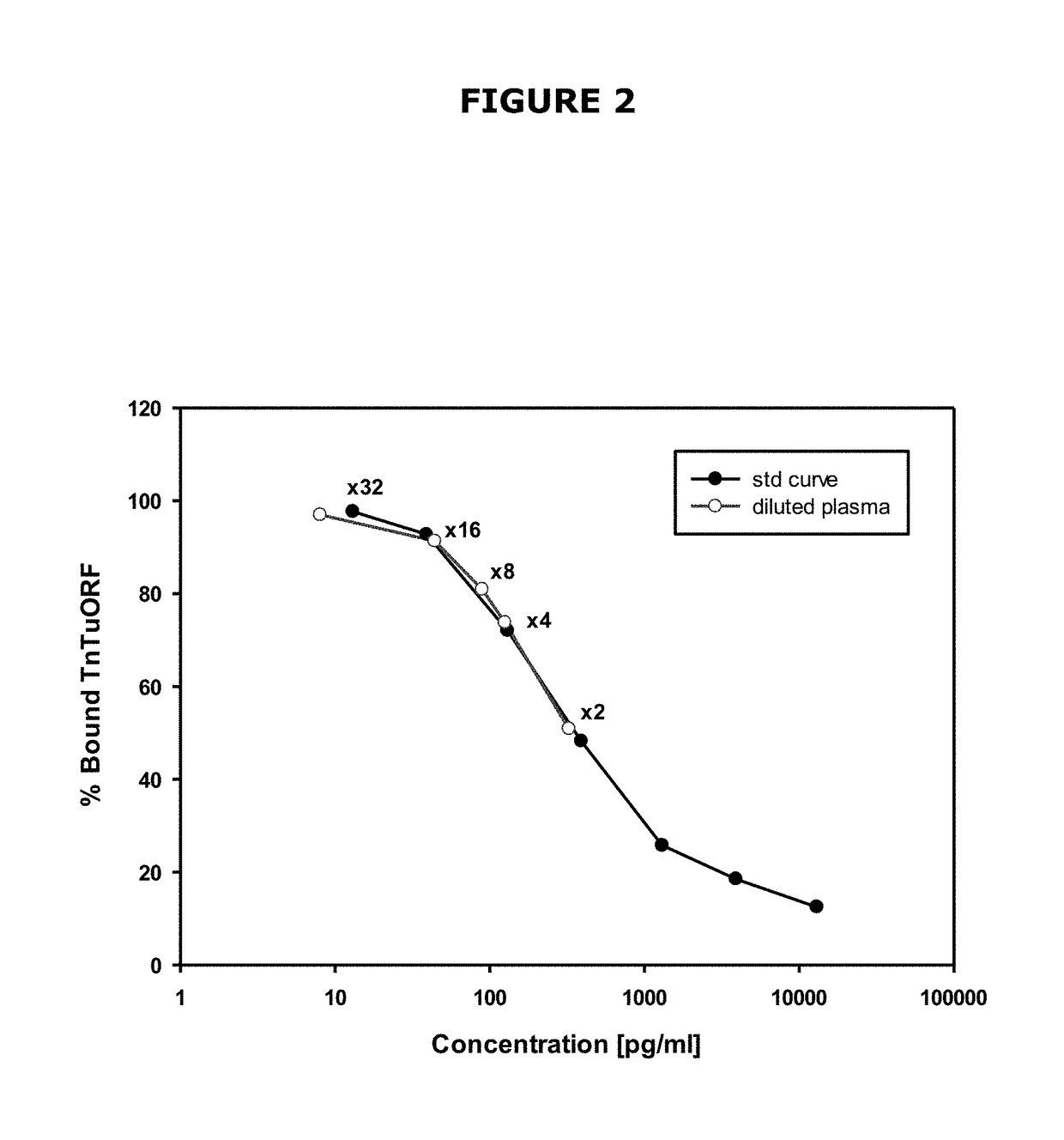
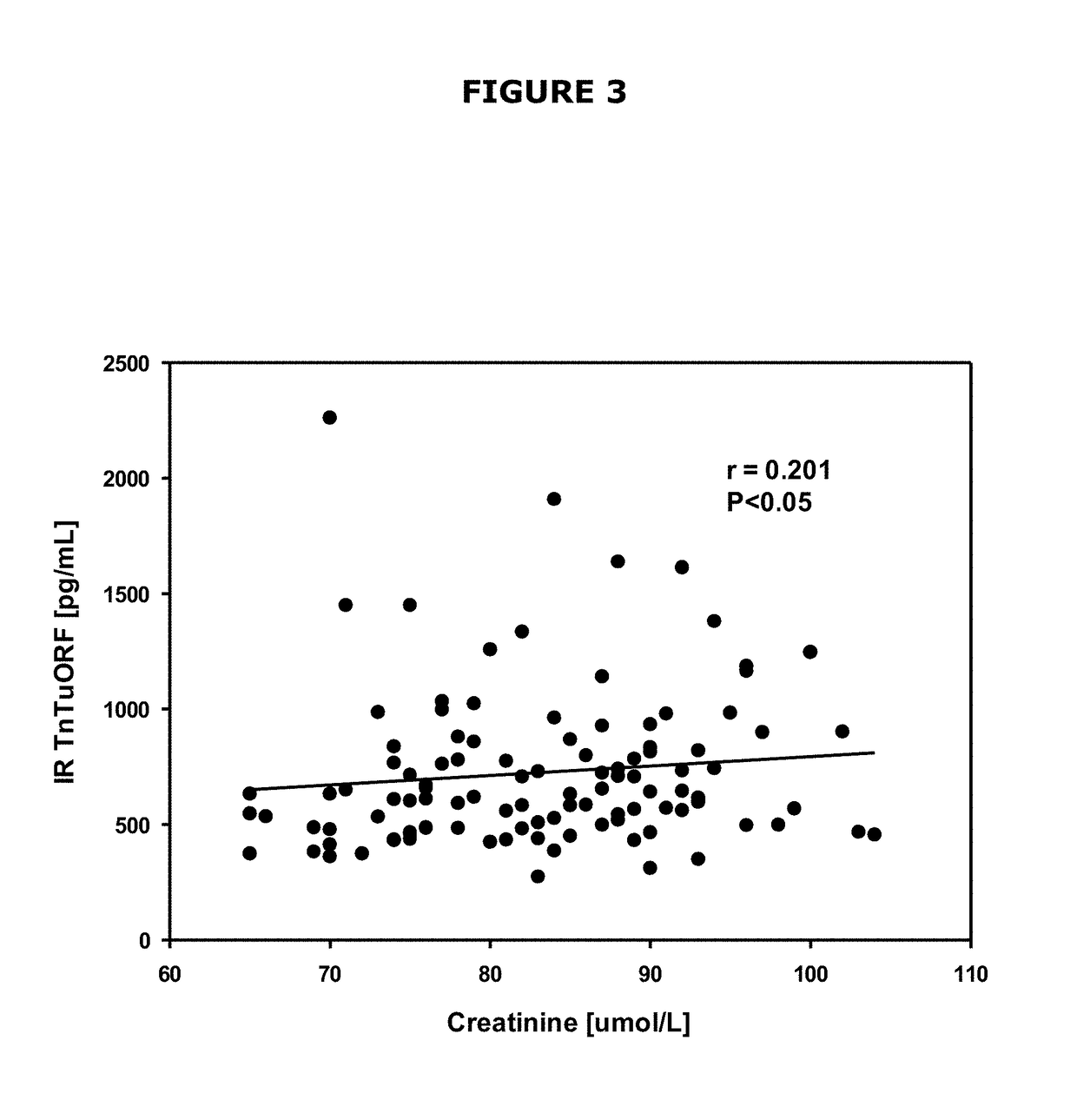
[0199]Polyclonal antibody r244 directed towards the amino terminus of TnTuORF yielded a sensitive, specific radioimmunoassay (FIG. 2), with a mean zero binding of 31±3%, sample detection limit of 26±6 pg / mL, ED50 of 458±105 pg / mL and a working range of 130-3900 pg / mL in which the intra-assay coefficient of variation (CV) was 3-EDTA and that it diluted in parallel with the synthetic standard curve (FIG. 2). Immunoreactive TnTuORF levels in normal human venous plasma (mean±SD) were 725±340 pg / mL (range 273-2260 pg / mL, 99th percentile of upper limit of normal range=1885 pg / mL); immunoreactivity was detected in every sample. TnTuORF concentrations were significantly higher in men (825±403 pg / mL vs. 666±285 pg / mL, P=0.018) and had a significant biochemical correlation only with plasma creatinine (r=0.201, P=0.036, FIG. 3). Concomitant hsTnT levels, age, blood pressures and BMI had no significant association with TnTuORF. Corresponding hsTnT leve...
example 3
ctive TnTuORF Release into the Circulation
[0200]The form of TnT used for the diagnosis of MI is thought to be cardiac specific (Thygessen et al. (2012) Circulation 126:2020-2035). In line with this, Applicants anticipated that TnTuORF levels would be higher in blood draining the heart (i.e. in cardiac coronary sinus samples) than in blood supplying the heart (i.e. arterial samples). Coronary sinus plasma tended to contain higher concentrations of immunoreactive TnTuORF, compared with simultaneously drawn arterial plasma samples (978.2±118.1 pg / mL vs. 908.7±88.9 pg / mL, Mean±SD, P=0.472, FIG. 4). However, elevated venous levels of TnTuORF were also observed across the hepatic vein, whereas there tended to be reduced TnTuORF levels across the femoral vein. In comparison, hsTnT levels were significantly elevated in cardiac coronary sinus plasma samples (13.1±2.7 pg / mL vs. 10.1±1.8 pg / mL, P=0.019). Renal venous levels of hsTnT were significantly lower compared with arterial levels (8.6±1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com