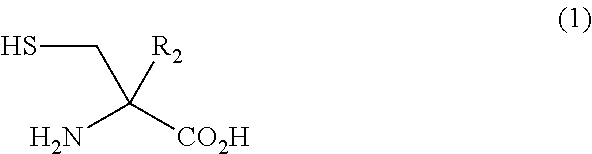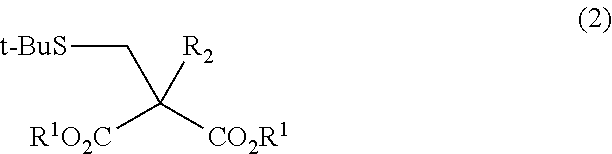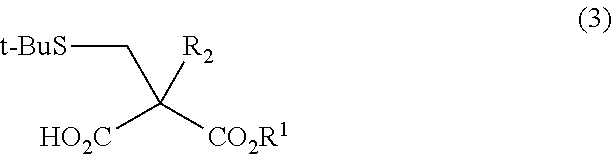Method for Producing Alpha-substituted Cysteine or Salt Thereof or Synthetic Intermediate of Alpha-substituted Cysteine
a technology of alpha-substituted cysteine and synthetic intermediate, which is applied in the field of producing alpha-substituted cysteine or salt thereof or synthetic intermediate of alpha-substituted cysteine, can solve the problems of low production efficiency, inconvenient industrial production method, and difficult removal of metal salt generated during reaction, etc., to achieve stable, industrial-scale production of -substituted cysteine, simple, quick and safe production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[Example 1] Step (viii): Production of tert-Butylthiomethanol
[0423]Under nitrogen gas flow, 240 g (2.66 mol) of tert-butyl mercaptan and 216 g (2.66 mol) of formalin (37 wt %) were placed in a 1-L reactor at room temperature, and the resulting mixture was stirred. After allowing the reaction to proceed at 60° C. for 9 hours, separation extraction was carried out by adding 160 g of cyclohexane while keeping the reactor at a temperature within the range of 50° C. to 60° C., followed by removing the aqueous phase. Thereafter, cyclohexane was removed by concentrating the cyclohexane layer under reduced pressure. The resulting crude tert-butylthiomethanol was subjected to distillation under reduced pressure at 70° C. / 2.0 kPa to obtain 208 g (1.73 mmol; yield, 65.0%) of tert-butylthiomethanol. As a result of GC analysis under , its purity was found to be 99% by area. The measurement results obtained were as follows.
[0424]1H-NMR (400 MHz, CDCl3) δ 1.41 (9H, s), 2.11 (1H, t, J=6.4 Hz), 4.83...
example 2
[Example 2] Step (ix): Production of tert-Butylthiochloromethane
[0425]Under nitrogen gas flow, 190 g (1.58 mol) of tert-butylthiomethanol produced in Example 1 and 1.44 kg of cyclohexane were placed in a 3-L reactor at room temperature. To the resulting mixture, 150 g (1.90 mol) of pyridine was added while the inner temperature was kept at 0° C. To the mixture, 226 g (1.89 mol) of thionyl chloride was then added dropwise while the inner temperature was kept at −2° C. to 6° C. After stirring the resulting mixture at a temperature within the range of −4° C. to 0° C. for 1 hour, 380 g of cyclohexane was added thereto, and the precipitated salt was removed by filtration. Cyclohexane was removed by concentrating the obtained filtrate by distillation, and the resulting crude tert-butylthiochloromethane was subjected to distillation under reduced pressure at 57° C. / 3.0 kPa to obtain 140 g (1.01 mol; yield, 63.9%) of tert-butylthiochloromethane. As a result of GC analysis under , its purity...
example 3
[Example 3] Step (x): Production of Diethyl 2-[(tert-Butylthio)methyl]-2-methylmalonate
[0427]Under nitrogen gas flow, 107 g (0.953 mol) of potassium-tert-butoxide and 790 g of tetrahydrofuran were placed in a 3-L reactor. To the resulting mixture, 158 g (0.907 mol) of diethyl methylmalonate was added dropwise at an inner temperature within the range of 18° C. to 26° C. for 30 minutes. After stirring the resulting mixture for 30 minutes, 132 g (0.953 mol) of tert-butylthiochloromethane produced in Example 2 was added dropwise thereto for 1 hour while the inner temperature was kept within the range of 18° C. to 26° C. After stirring the resulting mixture for 3 hours, 395 g of toluene and 395 g (0.229 mol) of 2 wt % hydrochloric acid were added thereto, followed by stirring the resulting mixture for 30 minutes. The aqueous layer was then removed by separation. Subsequently, an operation of adding 395 g of water to the toluene layer, stirring the resulting mixture for 1 hour, and then r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com