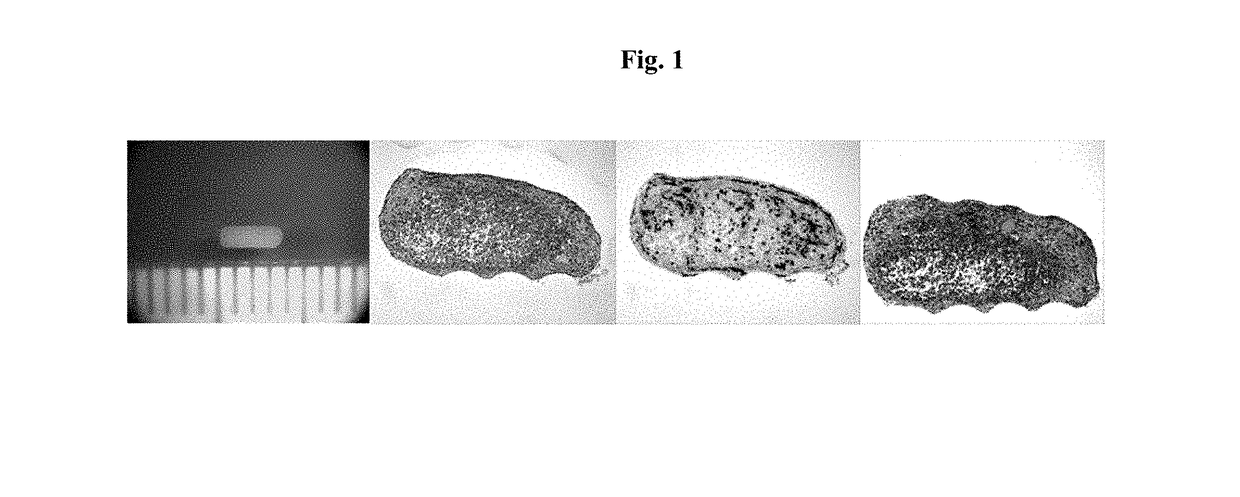
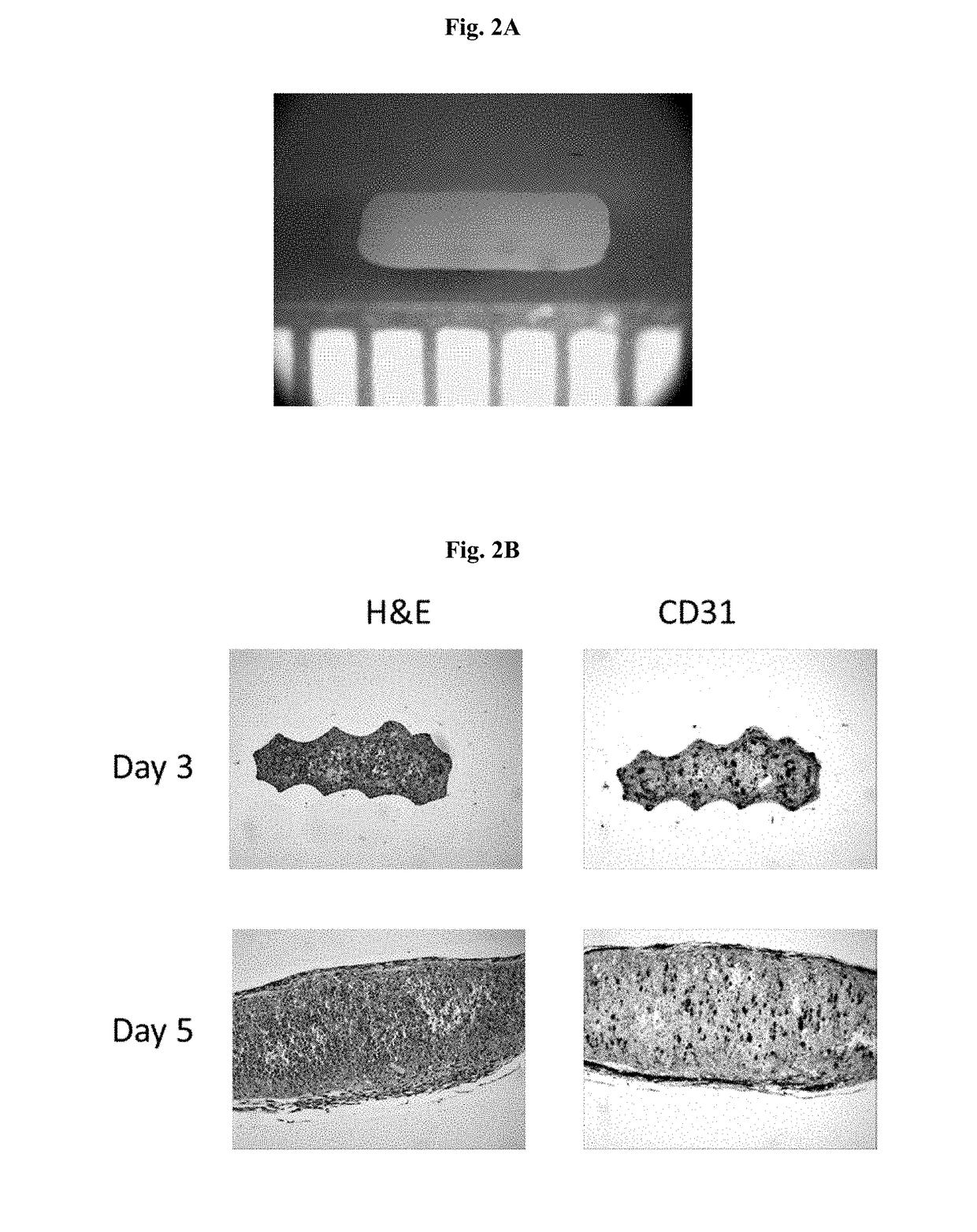
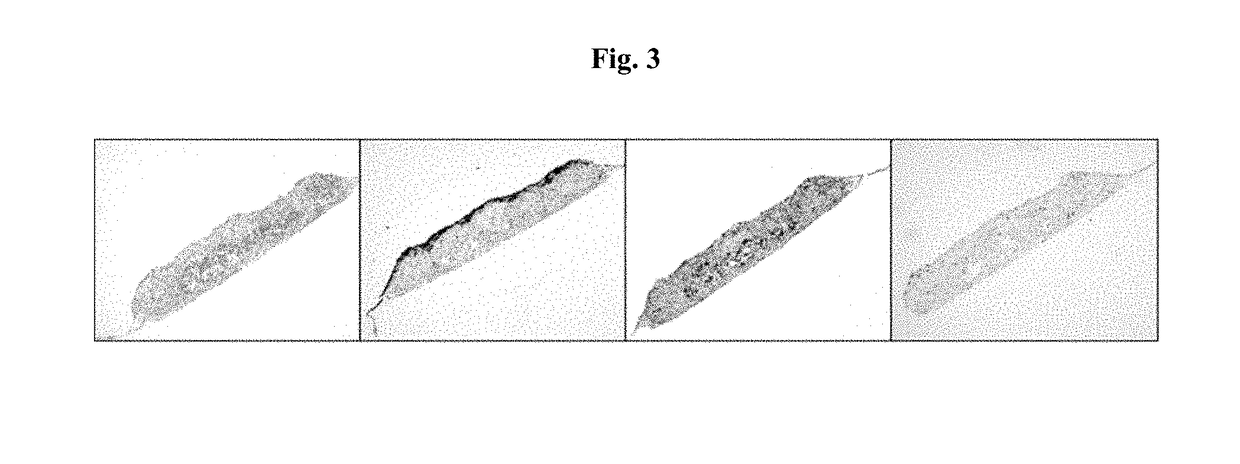
Engineered Tissues for in vitro Research Uses, Arrays Thereof, and Methods of Making the Same
a technology tissue, applied in the field of in vitro research tissue engineering tissues, arrays thereof, and methods of making the same, can solve the problems of low rate of new therapeutic discovery, unsustainable r&d costs, and the cost of drug discovery is approximately $1.8 billion, so as to increase the number and quality of innovative, cost-effective, and the effect of improving the quality of new therapeutic discovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ure
Smooth Muscle Cells
[0194]Primary human aortic smooth muscle cells (HASMC; GIBCO / Invitrogen Corp., Carlsbad, Calif.) were maintained and expanded in low glucose dulbecco's modified eagle medium (DMEM; Invitrogen Corp., Carlsbad, Calif.) supplemented with 10% fetal bovine serum (FBS), 100 U / mL Penicillin, 0.1 mg / mL streptomycin, 0.25μg / mL of amphotericin B, 0.01M of HEPES (all from Invitrogen Corp., Carlsbad, Calif.), 50 mg / L of proline, 50 mg / L of glycine, 20 mg / L of alanine, 50 mg / L of ascorbic acid, and 3 μg / L of CuSO4 (all from Sigma, St. Louis, Mo.) at 37° C. and 5% CO2. Confluent cultures of HASMC between passage 4 and 8 were used in all studies.
Endothelial Cells
[0195]Primary human aortic endothelial cells (HAEC; GIBCO / Invitrogen Corp., Carlsbad, Calif.) were maintained and expanded in Medium 199 (Invitrogen Corp., Carlsbad, Calif.) supplemented with 10% FBS, 1 μg / mL of hydrocortisone, 10 ng / mL of human epidermal growth factor, 3 ng / mL of basic fibroblast growth factor, 10 μg...
example 2
Solutions and Mold
[0200]Preparation of 2% and 4% (w / v) NovoGel™ Solution
[0201]1 g or 2 g (for 2% or 4% respectively) of NovoGel™ (Organovo, San Diego, Calif.) was dissolved in 50 mL of Dulbecco's phosphate buffered saline (DPBS; Invitrogen Corp., Carlsbad, Calif.). Briefly, the DPBS and NovoGel™ are heated to 85° C. on a hot plate with constant stirring until the NovoGel™ dissolves completely. NovoGel™ solution is sterilized by steam sterilization at 125° C. for 25 minutes. The NovoGel™ solution remains in liquid phase as long as the temperature is maintained above 65.5° C. Below this temperature a phase transition occurs, the viscosity of the NovoGel™ solution increases and the NovoGel™ forms a solid gel.
Preparation of NovoGel™ Mold
[0202]An NovoGel™ mold was fabricated for the incubation of cylindrical bio-ink using a Teflon® mold that fit a 10 cm Petri dish. Briefly, the Teflon® mold was pre-sterilized using 70% ethanol solution and subjecting the mold to UV light for 45 minutes. ...
example 3
on of HASMC-HAEC Polytypic Cylindrical Bio-Ink
[0203]To prepare polytypic cylindrical bio-ink, HASMC and HAEC were individually collected and then mixed at pre-determined ratios. Briefly, the culture medium was removed from confluent culture flasks and the cells were washed with DPBS (1 ml / 5 cm2 of growth area). Cells were detached from the surface of the culture flasks by incubation in the presence of trypsin (1 ml / 15 cm2 of growth area; Invitrogen Corp., Carlsbad, Calif.) for 10 minutes. HASMC were detached using 0.15% trypsin while HAEC were detached using 0.1% trypsin. Following the incubation appropriate culture medium was added to the flasks (2× volume with respect to trypsin volume). The cell suspension was centrifuged at 200 g for 6 minutes followed by complete removal of supernatant solution. Cell pellets were resuspended in respective culture medium and counted using a hemocytometer. Appropriate volumes of HASMC and HAEC were combined to yield a polytypic cell suspension co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com