Methods Of Selectively Treating Asthma Using IL-13 Antagonists
a selective treatment and asthma technology, applied in antibody medical ingredients, cleaning equipments, peptides, etc., can solve the problems of significant unmet medical needs and major global health burden of asthma, and achieve the effects of minimizing the risk of il-13 antagonism, reducing the frequency of asthma exacerbations, and maximizing the benefi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Il-13 Antagonists
[0169]For the purposes of the invention, IL-13 antagonists that compete with a specified anti-IL-13 antibody set forth herein, including antibody 01951 / G12 (SEQ ID NO:14 and 16) for binding to IL-13 can be identified by methods known in the art, and further as described in the following methodology.
All experiments are performed using a Biacore T200 instrument. Biacore T200 Control software and Biacore T200 Evaluation software are used for the control and analysis of experiments, respectively.
Part 1:
[0170]The anti-human IL-13 antibody ANTIBODY 01951 / G12 is immobilized to a CMS sensorchip surface using the amine coupling method. Briefly, the surface of the measuring flow cell is activated with EDC / NHS, followed by a 700 second addition of 50 μg / mL ANTIBODY 01951 / G12 in 10 mM sodium acetate pH 4.5. Based on previous experiments, these buffers and injection times are sufficient to allow saturation of the chip surface with ANTIBODY 01951 / G12, though exp...
example 2
Identification of the Antibody 01951 / G12 Epitope Residues:
Peptide Mapping:
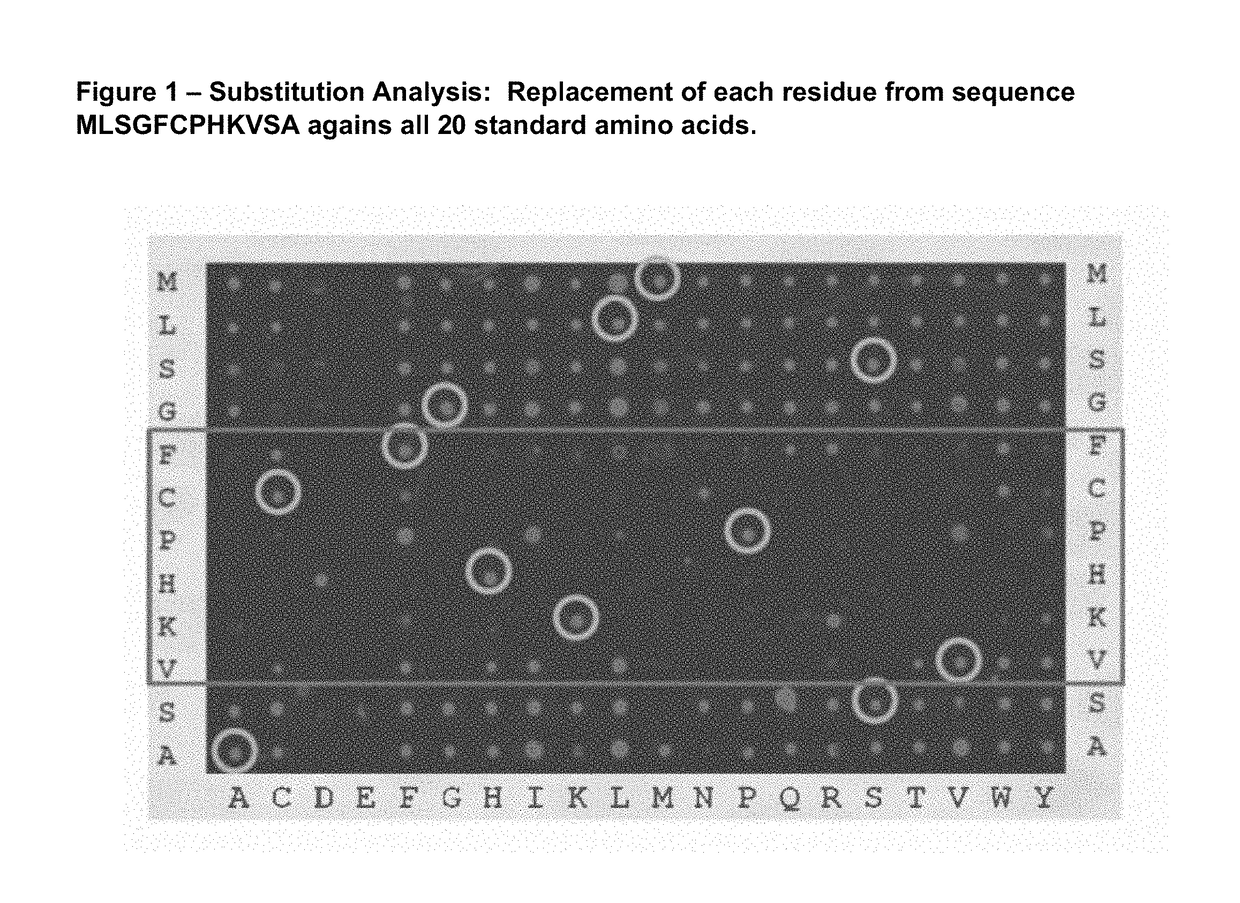
[0173]The sequence of IL-13 was probed at the peptide level to identify the binding site with ANTIBODY 01951 / G12. Thirty four 15-mer peptides were synthesized in order to scan the entire sequence with 12 residue overlap starting from the N-terminus. The synthesis of the peptides, preparation of the peptide-array slides, incubation with antibodies and data analysis where done as described by Maksimov et al. (PLoS ONE 7(3): e34212).
[0174]The results are shown in Table 4. The binding signal intensity (light unit) observed is reported for each peptide, in column 1 against a control unrelated human antibody and in column 2 against ANTIBODY 01951 / G12. Signals above 10000 Light Units are considered as being significant. 2 overlapping peptides in the set of the 34 peptides produced a signal above that threshold, peptide 22 (TQRMLSGFCPHKVSA) (SEQ ID NO: 31) and peptide 23 (MLSGFCPHKVSAGQF) (SEQ ID NO: 32). The overlapp...
example 3
Pharmacogenetic Analysis of IL4-Rα SNPs and Discovery of AIR Markers 1-9:
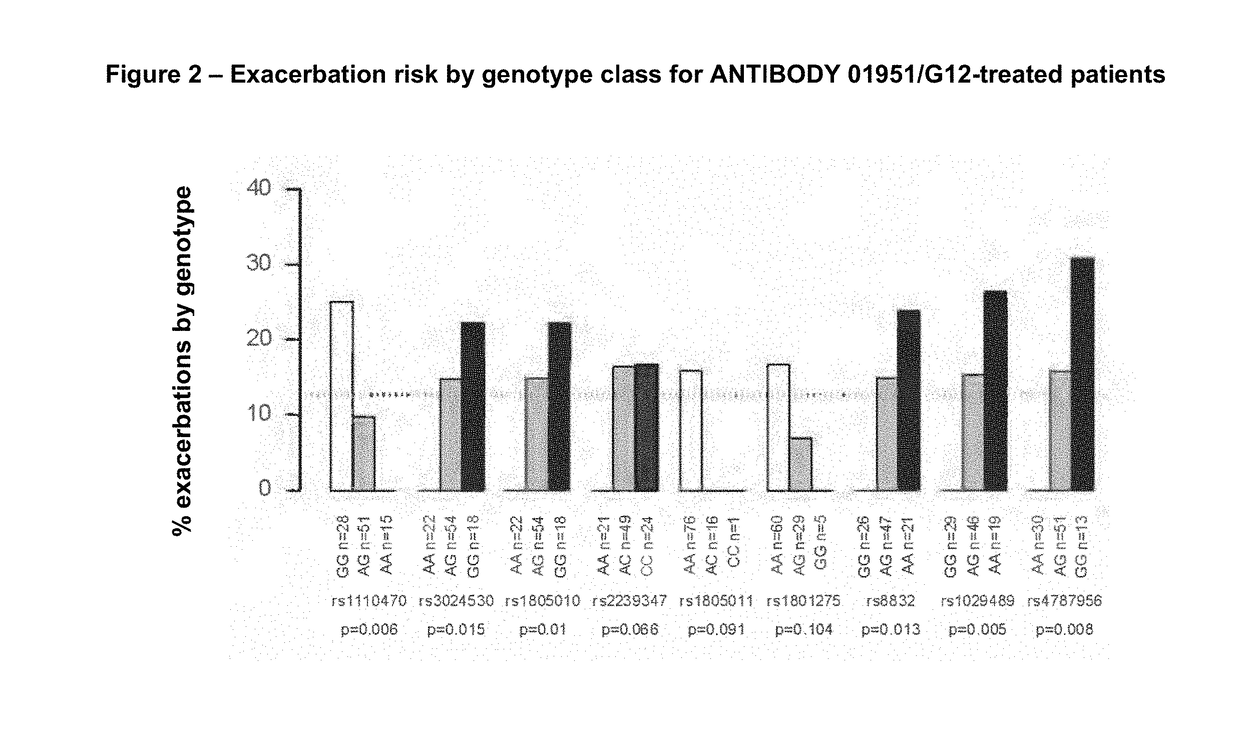
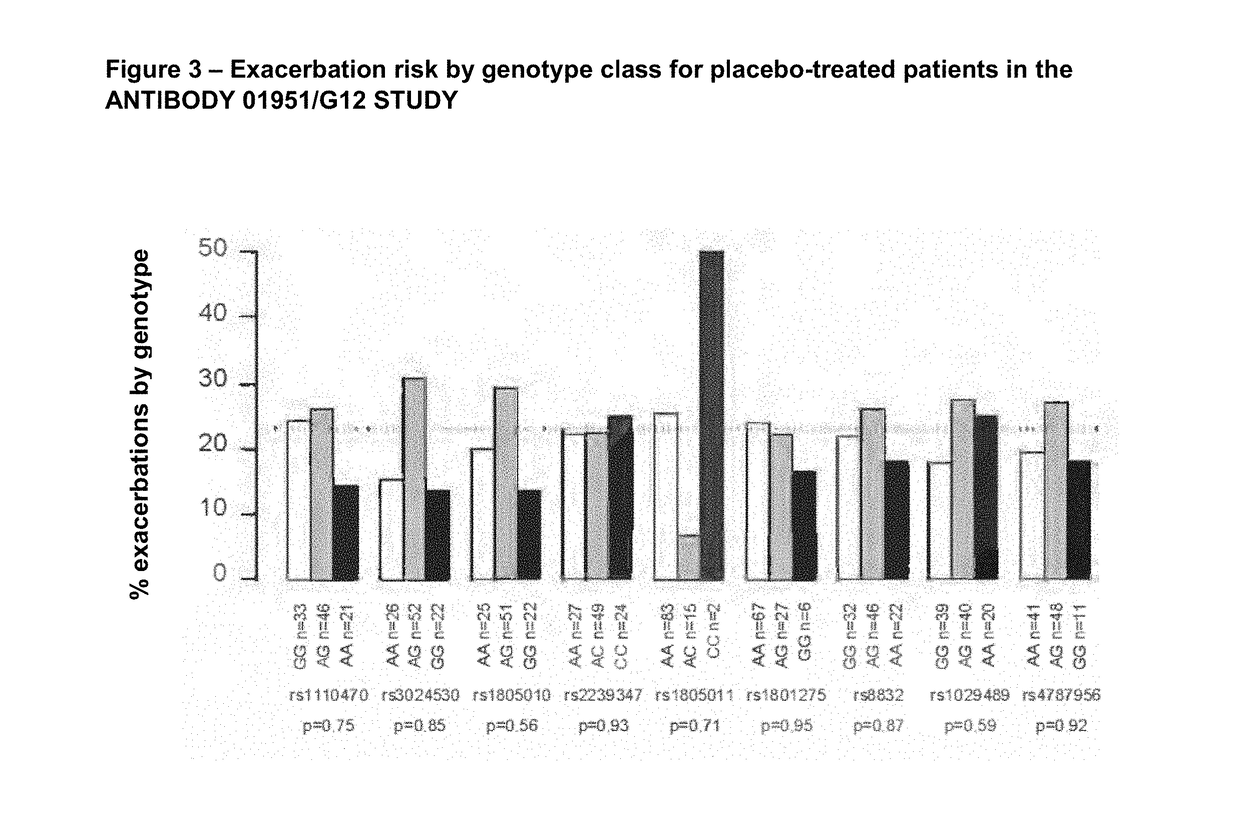
[0176]ANTIBODY 01951 / G12 is a fully human IgG1 / κ monoclonal antibody. CQAX576A2207 was a Phase II clinical study to evaluate the effectiveness and safety of ANTIBODY 01951 / G12 in patients with moderate to severe persistent asthma following multiple doses, when added to existing asthma therapy.
[0177]To assess associations of several single nucleotide polymorphisms (SNPs) in the IL4-Rα gene and risk of asthma exacerbation in patients treated with ANTIBODY 01951 / G12, DNA was collected from patients enrolled in the CQAX576A2207 trial and nine SNPs in the IL4-Rα gene were genotyped in these samples. Statistical tests of association were performed to assess the evidence for association of SNPs in the IL4-Rα gene with risk of asthma exacerbation, as well as with other asthma clinical endpoints, in patients treated with ANTIBODY 01951 / G12.
[0178]In this manner, a pharmacogenetic analysis was undertaken, with the objecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com