Treating diabetes with genetically modified beta cells
a technology of genetically modified beta cells and insulin, applied in the field of genetically modified human beta cells, can solve the problems of beta cell malfunction, failure of beta cell transplantation, de-differentiation, etc., and achieve the effects of improving the activity of the agent, enhancing the binding to an extracellular matrix, and increasing the circulating half-li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ls Used to Assess Expression Levels of CXCL12-a and -b Isoforms
[0118]HEK293 cells were transfected with 2 different isoforms of CXCL12 (alpha and beta) using commercially available plasmids for each isoform (plasmids available from GenScript). Transfected cells were selected with 250 ug / mL of G418 (commercially available from ThermoFisher) and a stable pool for each isoform was created. Cells were allowed to condition a suitable medium for 3 days. Conditioned medium from the transfected HEK293 cells expressing CXCL12 alpha and CXCL12 beta were diluted 1:1 with assay dilution buffer. Two separate pools were established for each isoform and then the concentration of each isoform in solution were obtained by absorption using a standardized concentration curve. This experiment was repeated twice and the results are as follows:
CXCL12 alphaCXCL12 beta1.310 nM1410 nM2.274 nM1330 nM
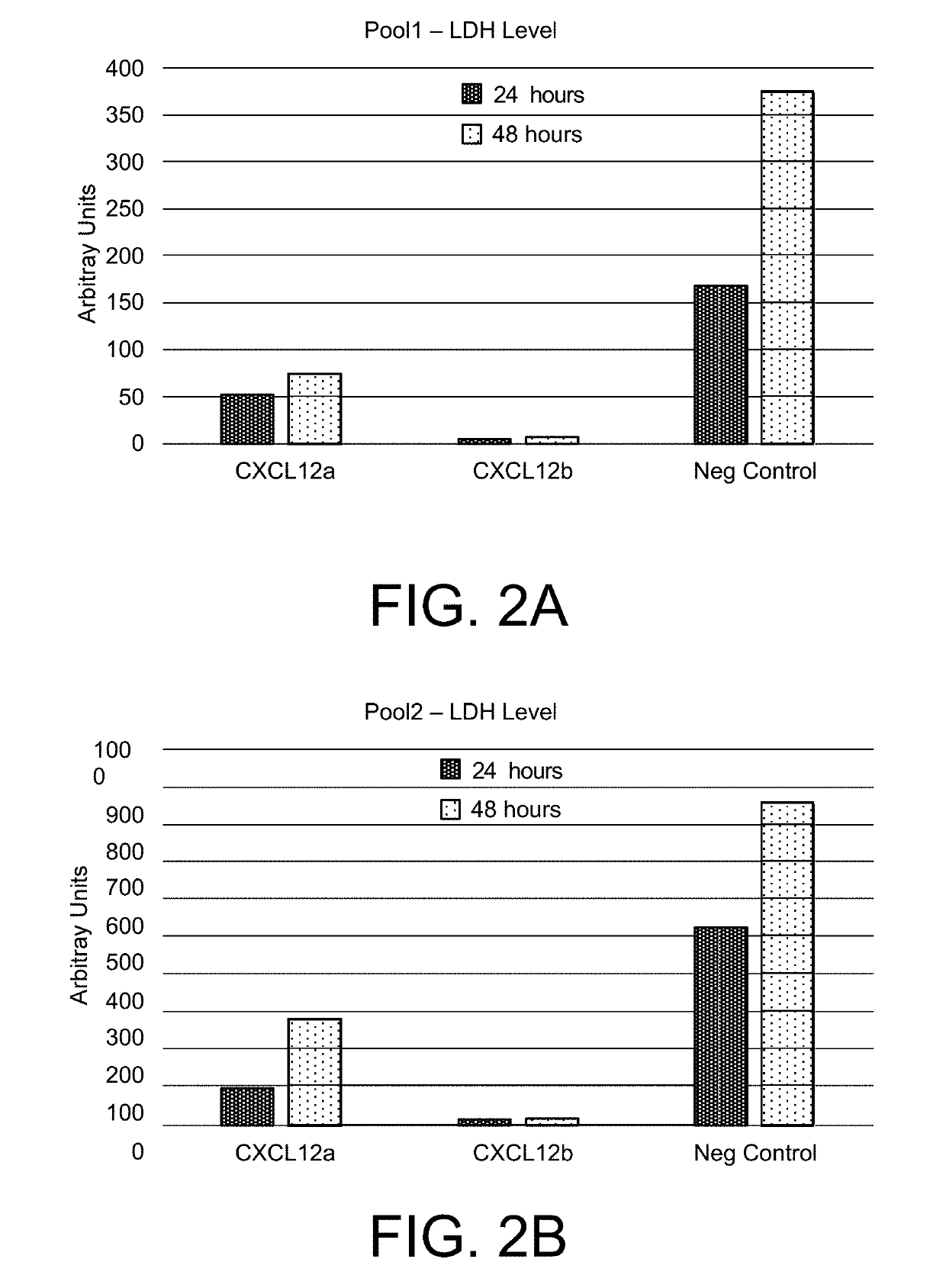
[0119]The above results evidence that transgenic model cells express CXCL12 beta at significantly higher level...
example 2
ls Used to Assess Expression Levels of Other Isoforms of CXCL12
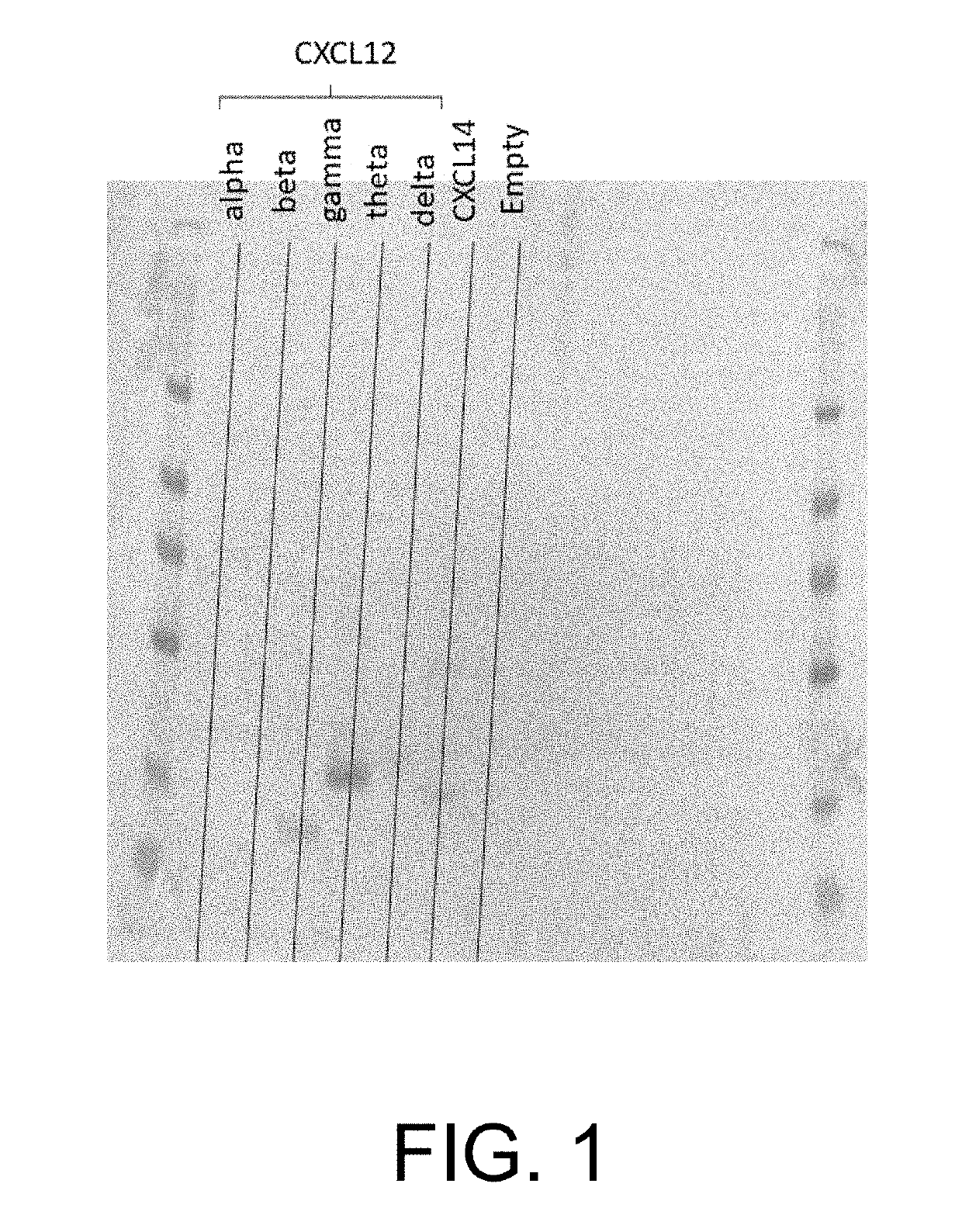
[0120]HEK293 cells were transfected with 5 different isoforms of CXCL12 (alpha and beta) using commercially available plasmids for each isoform (plasmids available from GenScript). Transfected cells were selected with 250 ug / mL of G418 (commercially available from ThermoFisher) and a stable pool for each isoform was created. Cells were allowed to condition in a suitable medium for 3 days. The conditioned medium was separated in a 4-8% NuPage gel (commercially available from ThermoFisher) with MES buffer and transferred to nitrocellulose (iBLOT).
[0121]Expression levels were detected with HRP labeled, anti-FLAG tag antibody / TMB chromogen (available from GenScript) on a Western Blot, as shown in FIG. 1. The results evidenced that the gamma, delta and theta isoforms of CXCL12 had greater concentrations than the alpha or beta isoforms.
example 3
on of Transgenic Beta Cells
[0122]Pancreatic beta cells derived from human induced pluripotent stem cells were purchased from Takara Bio USA, Inc. (Mountain View, Calif.) and cultured according to provided instructions.
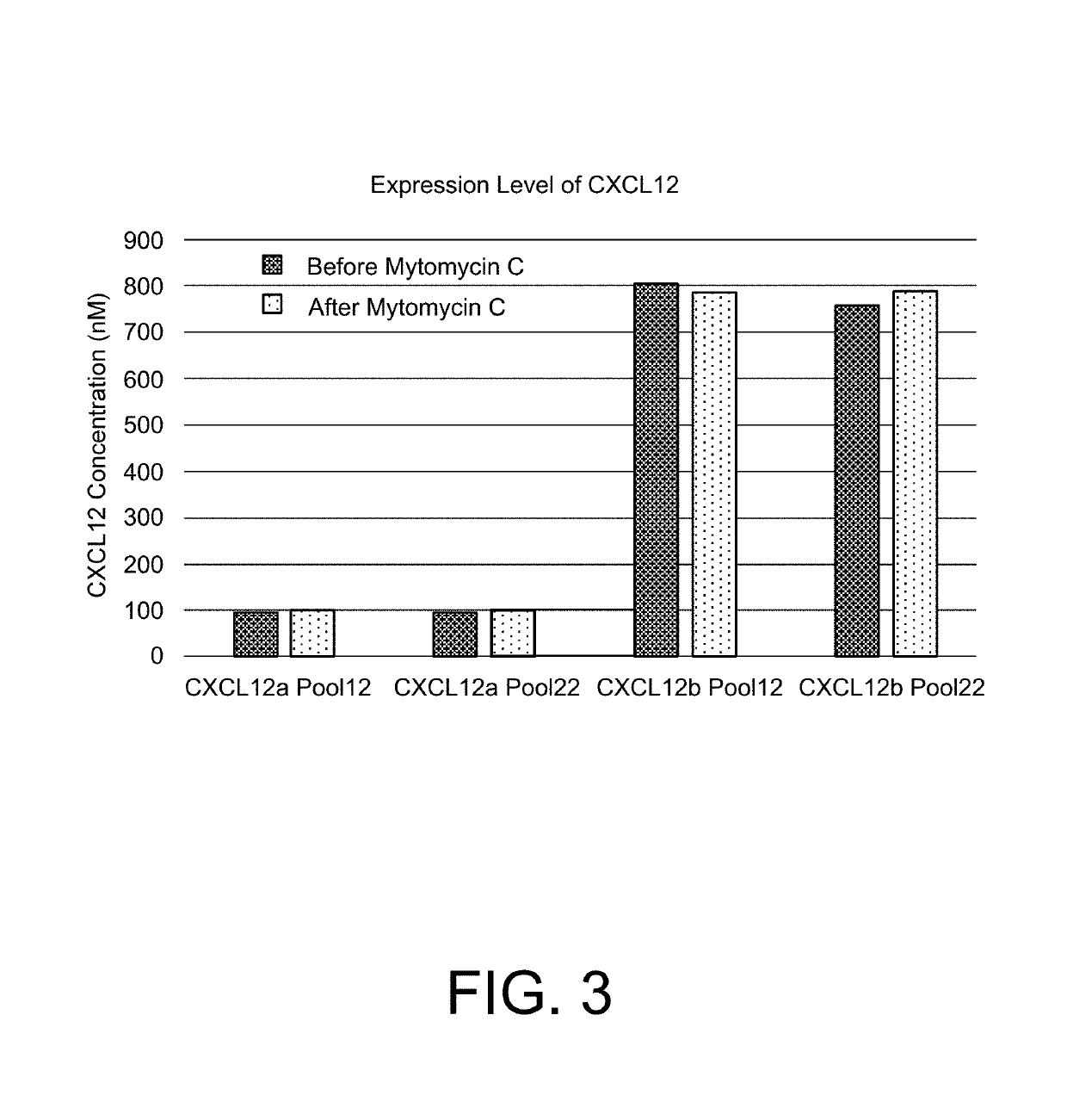
[0123]Cells were transduced with lentiviral vectors (pLenti-C-Myc-DDK, OriGene Technologies, Rockville, Md.) containing a human CXCL12 isotype (CXCL12a / SDF-1alpha or CXCL12b / SDF-1beta) or control. The lentiviral vectors were used at a ratio of about 10:1 per beta cell. The sequences, including the tag (underlined) are provided below. Concentration of the CXCL12 isotype was determined by ELISA (RayBioTech, Norcross, Ga.) (Table 1).
CXCL12a (aka SDF1a)Accession No. NM_199168SEQ. ID NO.: 9ATGAACGCCAAGGTCGTGGTCGTGCTGGTCCTCGTGCTGACCGCGCTCTGCCTCAGCGACGGGAAGCCCGTCAGCCTGAGCTACAGATGCCCATGCCGATTCTTCGAAAGCCATGTTGCCAGAGCCAACGTCAAGCATCTCAAAATTCTCAACACTCCAAACTGTGCCCTTCAGATTGTAGCCCGGCTGAAGAACAACAACAGACAAGTGTGCATTGACCCGAAGCTAAAGTGGATTCAGGAGTACCTGGAGAAAGCTTTAAACAAGACGCGTACGCGGCCGCTCGAGC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com