Soluble honokiol derivatives
a technology of honokiol and derivatives, applied in the field of compounds, compositions and methods of treatment or prevention, can solve the problems of brain cell ischemia or death or injury, unsuitable for clinical use in the treatment of ischemic stroke, etc., and achieve the effect of reducing edema and diminishing brain damag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Example 1 Preparation of Tetrabenzyl (3′,5-diallyl-[1,1′-biphenyl]-2,4′-diyl) bis(phosphate) (2) (HP-TMU)
[0051]
[0052]A solution of N-chlorosuccinimide (5.0 equiv) in CH3CN was heated at 40° C. for 5 min. A solution of dibenzyl phosphite (5.0 equiv) in CH3CN was added to this prepared solution and stirred at room temperature for 4 hours. Meanwhile, a solution of honokiol (1, 1 equiv), N,N-diisopropylethylamine (DIPEA, 5.0 equiv), and 4-dimethylaminopyridine (DMAP, 0.2 equiv) in CH3CN was prepared and added to the stirring mixture at room temperature. The resulting mixture was stirred at room temperature for 1 hour. The reaction mixture was quenched by H2O and extracted with toluene. The organic layer was collected and dried to afford tetrabenzyl (3′,5-diallyl-[1,1′-biphenyl]-2,4′-diyl) bis(phosphate) (referred as compound 2).
example 2
Preparation of Sodium 3′,5-diallyl-[1,1′-biphenyl]-2,4′-diyl bis(phosphate) (3)
[0053]
[0054]Bromotrimethylsilane was added to a solution of compound 2 (1 equiv) in CH2Cl2 was added bromotrimethylsilane under ice bath and stirred at room temperature for 3 hours. The reaction mixture was quenched by H2O and extracted with ethyl acetate. The aqueous layer was collected and dried to afford a crude product. The resulting residue was dissolved in EtOH and then sodium methoxide (4.4 equiv) was added. After stirring at room temperature for 18 hours, the organic solvent was removed in vacuum. The resulting residue was dissolved in H2O and extracted with ethyl acetate. The aqueous layer was collected and dried to afford sodium 3′,5-diallyl-[1,1′-biphenyl]-2,4′-diyl bis(phosphate) (referred to as compound 3).
example 3
HP-TMU Exerts (1) Superior Neuroprotective Effects Against Embolic Stroke as Compared to Honokiol (2) without Causing the Risk of Hemorrhagic Incidence in Mice
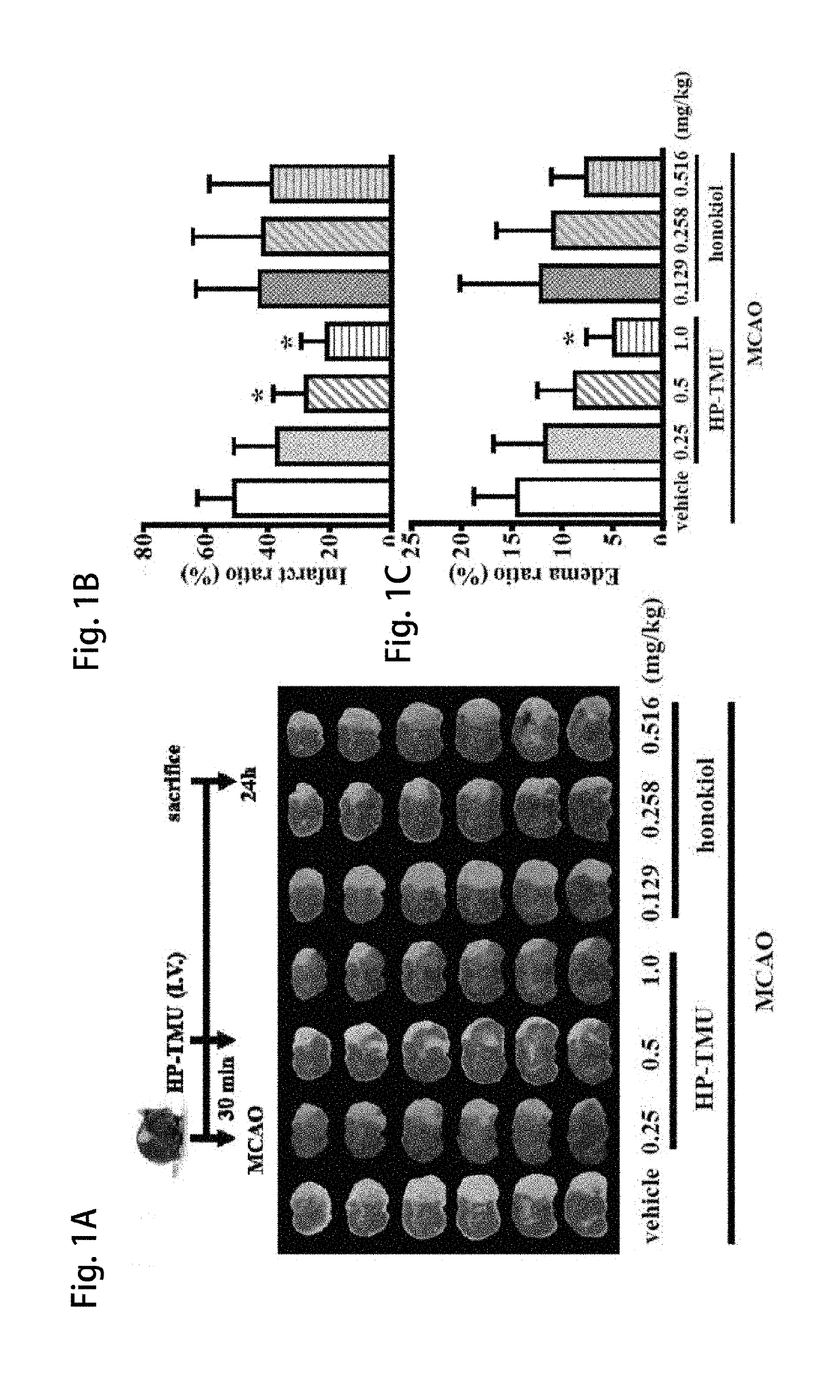
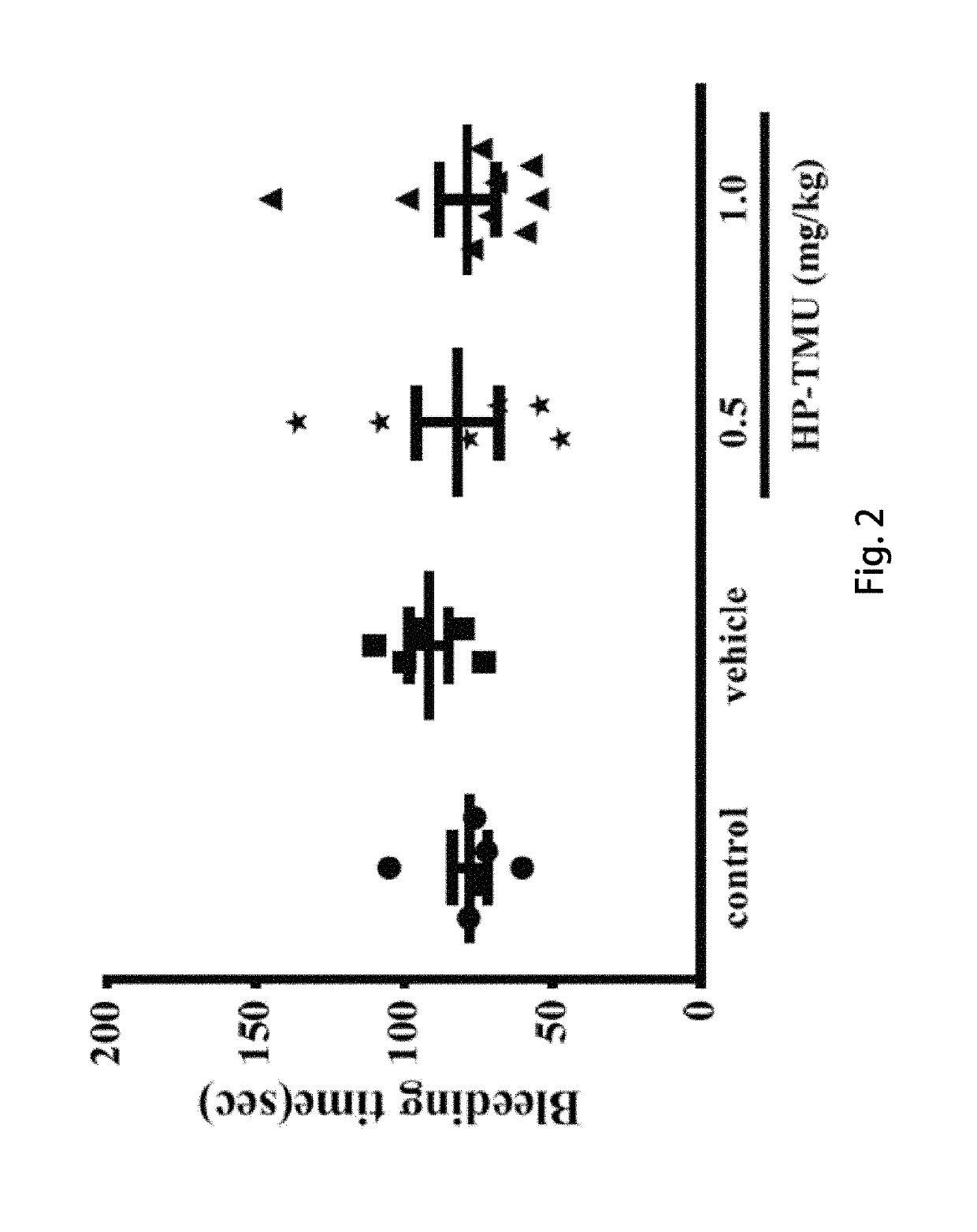
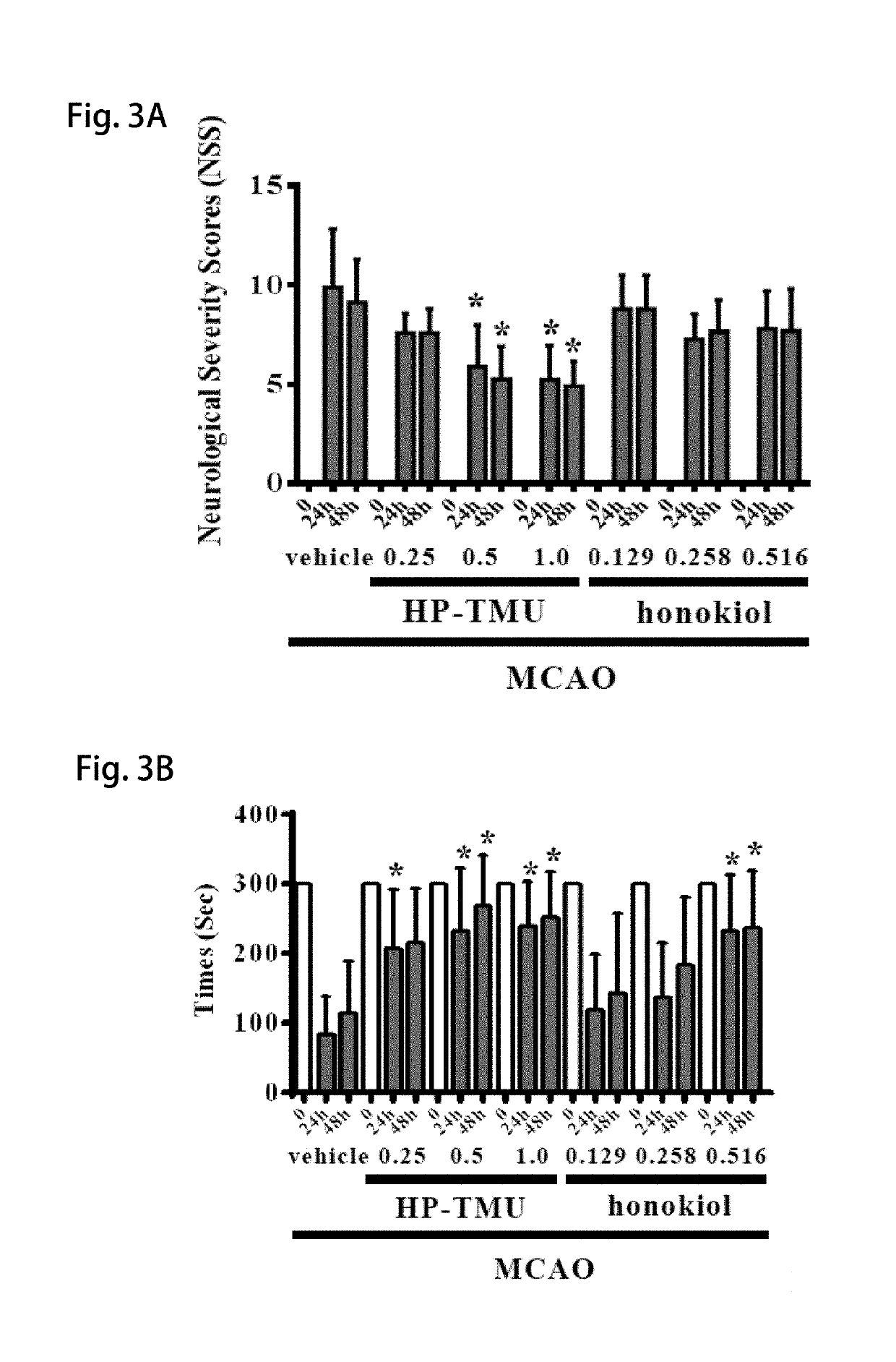
[0055]Coronal sections of 2,3,5-triphenyltetrazolium chloride (TTC)-stained brains were taken after middle cerebral artery occlusion (MCAO) in the vehicle group treated with an isovolumetric solvent (normal saline, intravenous [i.v.]), and groups treated with HP-TMU (0.25, 0.5 and 1 mg / kg, i.v.) or honokiol (0.129, 0.258 and 0.516 mg / kg, i.v.; the doses of honokiol were adjusted by the ratio of the molecular weight of honokiol to HP-TMU (266.33 / 514.22)) after 30 min-embolic occlusion. The densitometric analyses for the measurement of infarct volume and brain edema were performed after treatment with HP-TMU or honokiol against embolic stroke in mice. Bleeding time was measured 10 min after the i.v. administration of normal saline (isovolumetric control) or HP-TMU (0.5 and 1 mg / kg, i.v.) for 30 min.
[0056]As shown in FIG. 1A, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com