Medicament for treatment of liver cancer
a liver cancer and medicine technology, applied in the field of medicine for liver cancer, can solve the problems of high treatment cost, and achieve the effect of increasing the effect of sorafenib on liver cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Treatment of Liver Cancer In Vivo
[0045]As a representative of a human patient having liver cancer, a genetically manipulated mouse model (p19Arf− / −) was generated, in which liver cancers were induced by a constitutive expression of the oncogenic NrasG12V mutant.
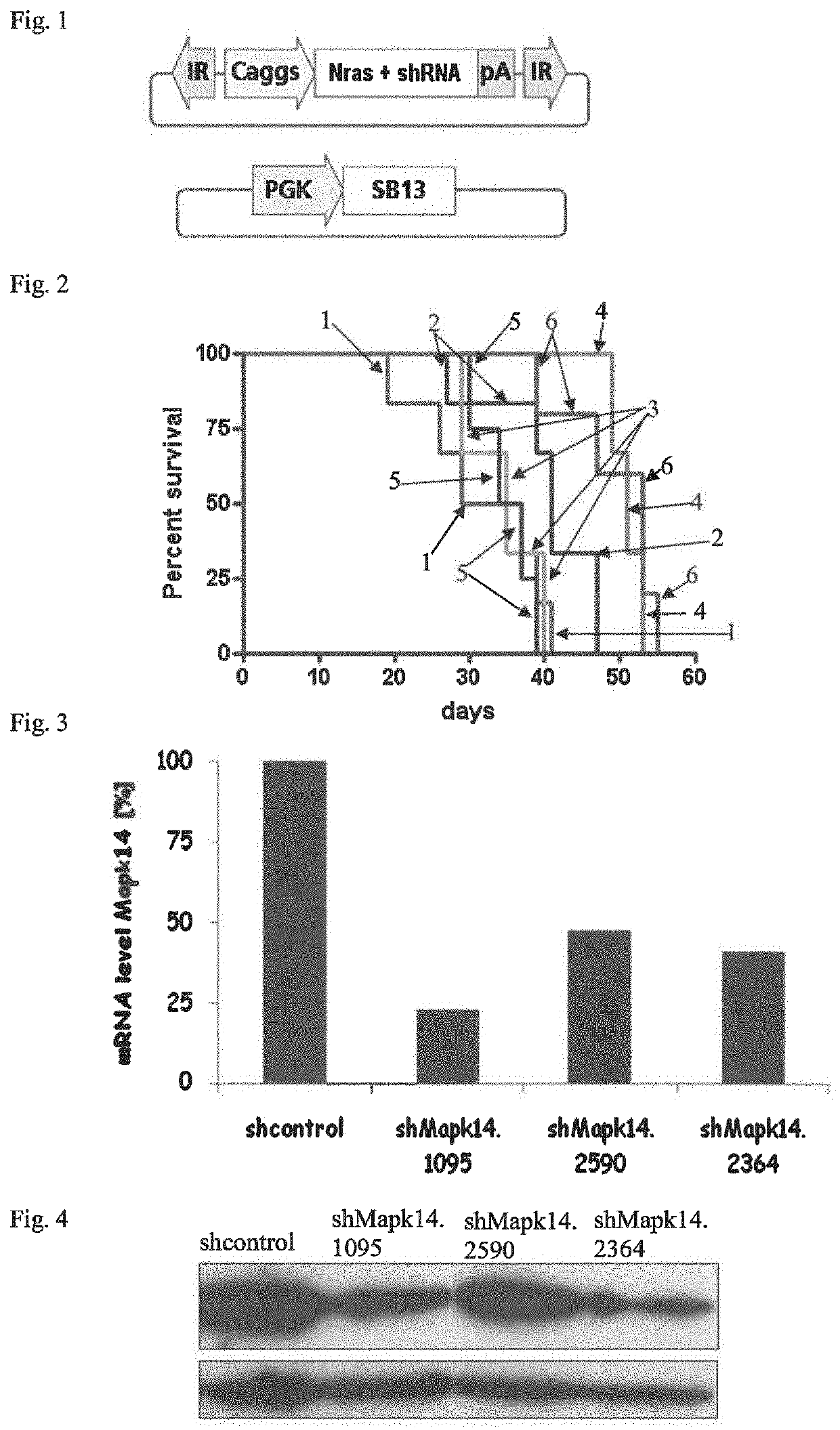
[0046]The nucleic acid construct for generating liver carcinomas is schematically shown in FIG. 1, containing an expression cassette, wherein the coding sequence for NrasG12V is arranged between a 5′ promoter sequence and a 3′ polyadenylation site, which expression cassette is flanked by two inverted repeat elements (IR). For intrahepatic delivery of the expression cassette, this nucleic acid construct was administered in combination with a nucleic acid construct containing an expression cassette for the transposase sleeping beauty 13 (SB13) under the control of the constitutive phosphoglycerate kinase promoter (PGK). The mouse model receiving both nucleic acid constructs shown in FIG. 1 was p19Arf− / −, providing the genetic b...
example 2
Treatment of Liver Cancer
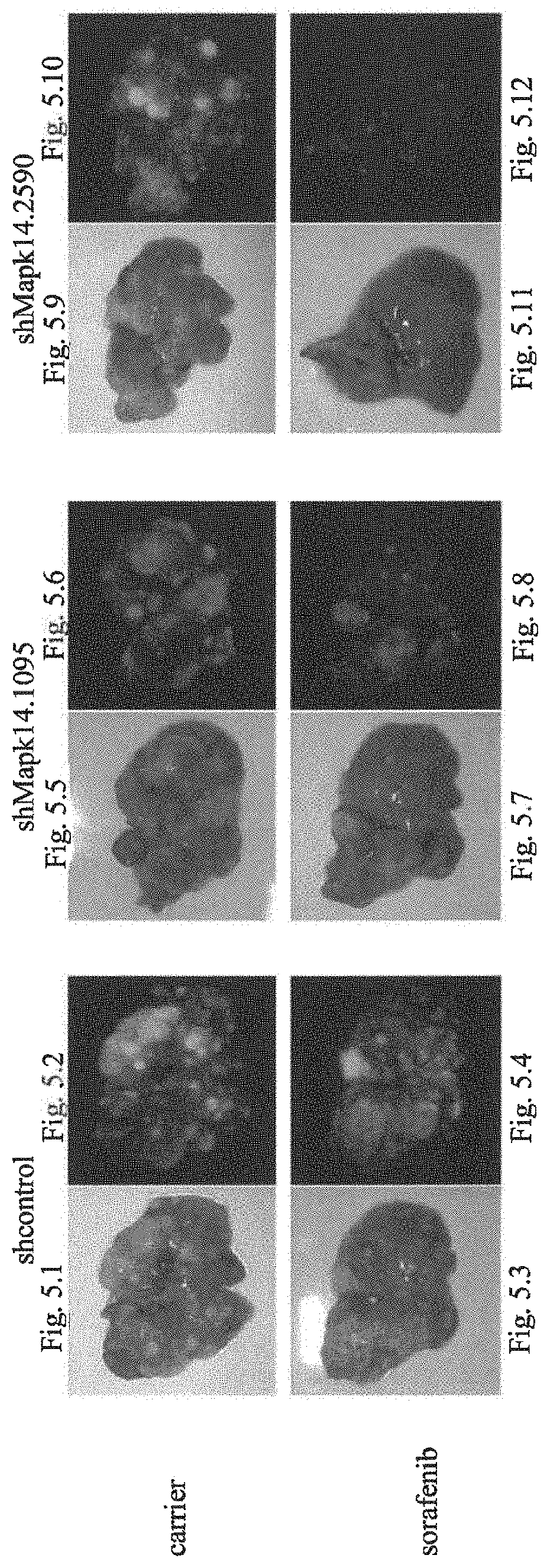
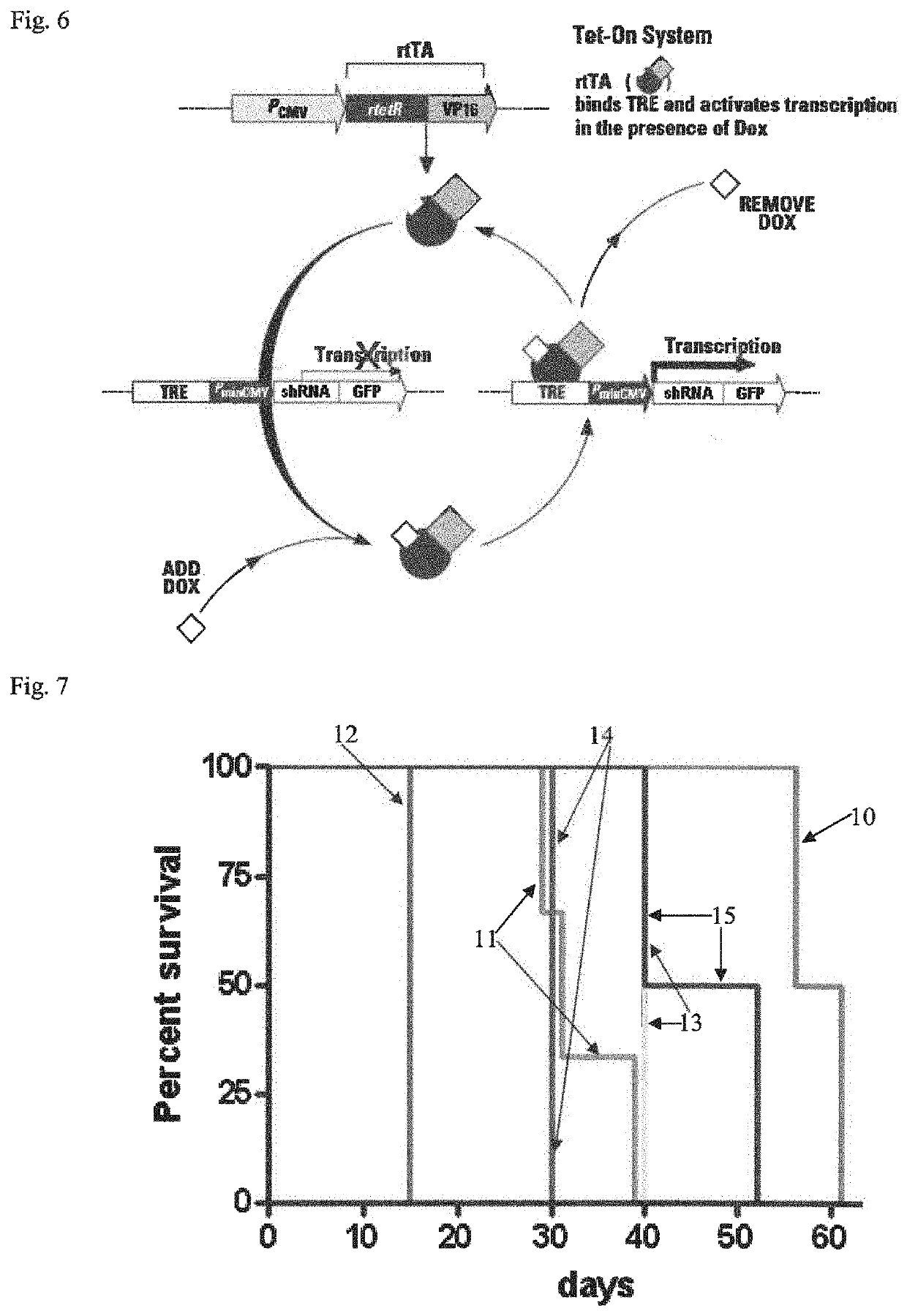
[0057]Again, mouse models were used for representing human liver cancer patients in treatment using Sorafenib in combination with an agent inhibiting the activity of Mapk14. As an example for a pharmaceutical agent for the inactivation of the activity of Mapk14, an shRNA (shMapk14.1095) specific for the murine mRNA encoding Mapk14 was used. The shRNA was provided by controlled expression using an expression cassette which is under the control of a tetracycline response element (TRE) of the otherwise constitutive viral promoter (PminCMV). The expression cassette contains the coding sequence for a shRNA molecule and the coding sequence of a GFP marker gene. The nucleic acid construct containing the expression cassette for the shRNA molecule and the coding sequence of a GFP marker gene under the control of the Tet-inducible promoter also contains a constitutive expression cassette for rtTA (rtetRVP16), which in the presence of Doxycycline (DOX) binds to the TRE...
example 3
Inactivation of Mapk14 by a Specific Kinase Inhibitor Increases the Efficacy of Sorafenib Against Liver Cancer Cell Lines
[0061]As an example for a medicament containing Sorafenib in combination with an inhibitor specific for Mapk14 kinase, BIRB-796, SB 202190 and SX 001 were tested in an in vitro assay. These in vitro experiments show the increased efficacy of Sorafenib when administered in combination with an inhibitor of Mapk14 kinase protein on the example of cultivated murine and human liver cancer cell lines.
[0062]In the assay, murine liver cancer cells (Nras arf− / −) were cultivated. Cultivated cells were treated with 10 μM Sorafenib, 2 μM BIRB, 12 μM BIRB and with a combination of 10 μM Sorafenib+2 μM BIRB, or a combination of 10 μM Sorafenib+12 μM BIRB. For each of these assays, a parallel assay was made, replacing Sorafenib or Mapk14 inhibitors by formulation agents without pharmaceutical active agent (carrier).
[0063]FIG. 8 shows the results of the viable cell count after tw...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com