Oxidative coupling of methane at near ambient feed temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Incremental Change of CH4:O2 Molar Ratio and Feed Temperature with a La2O3 / CeO2 Catalyst
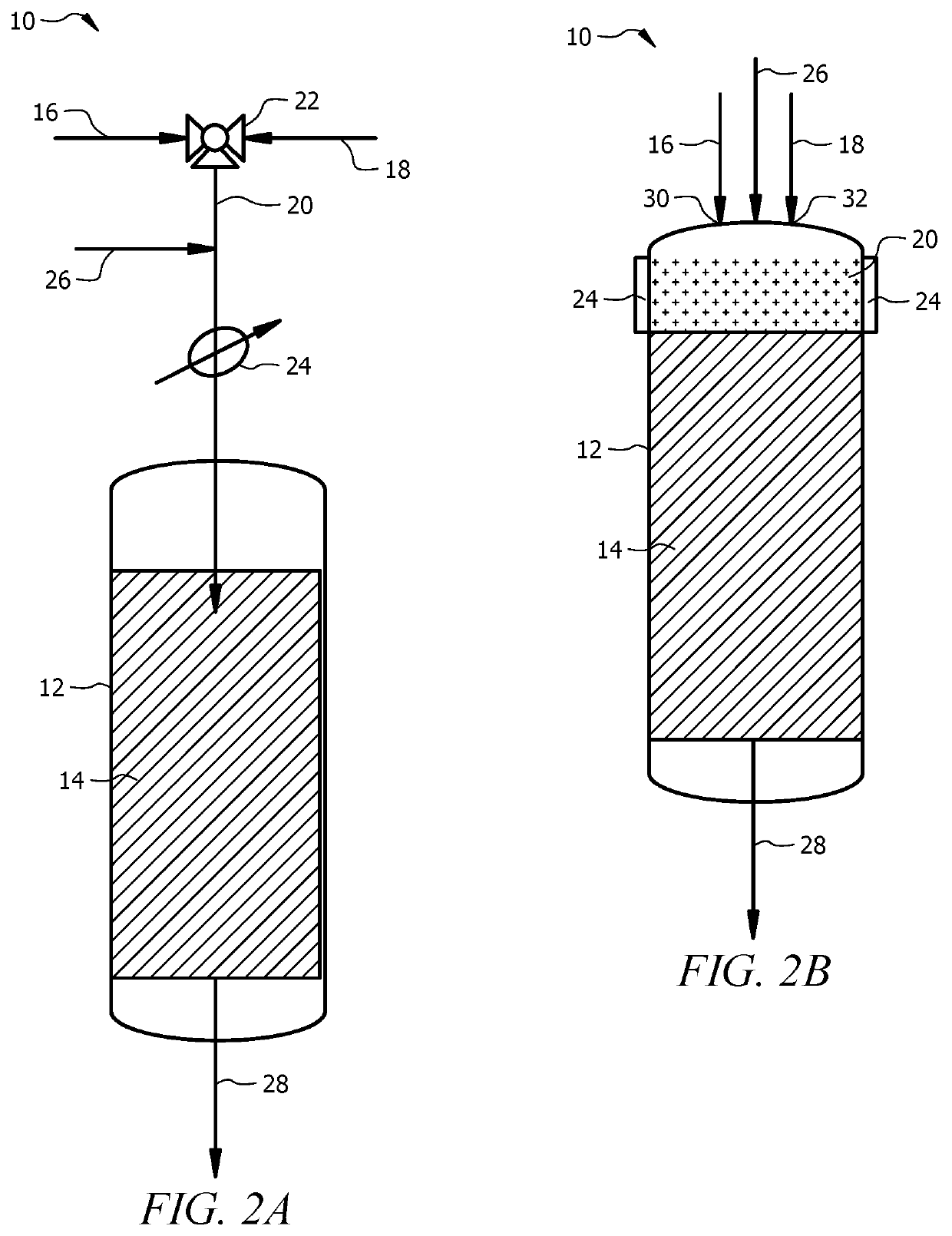
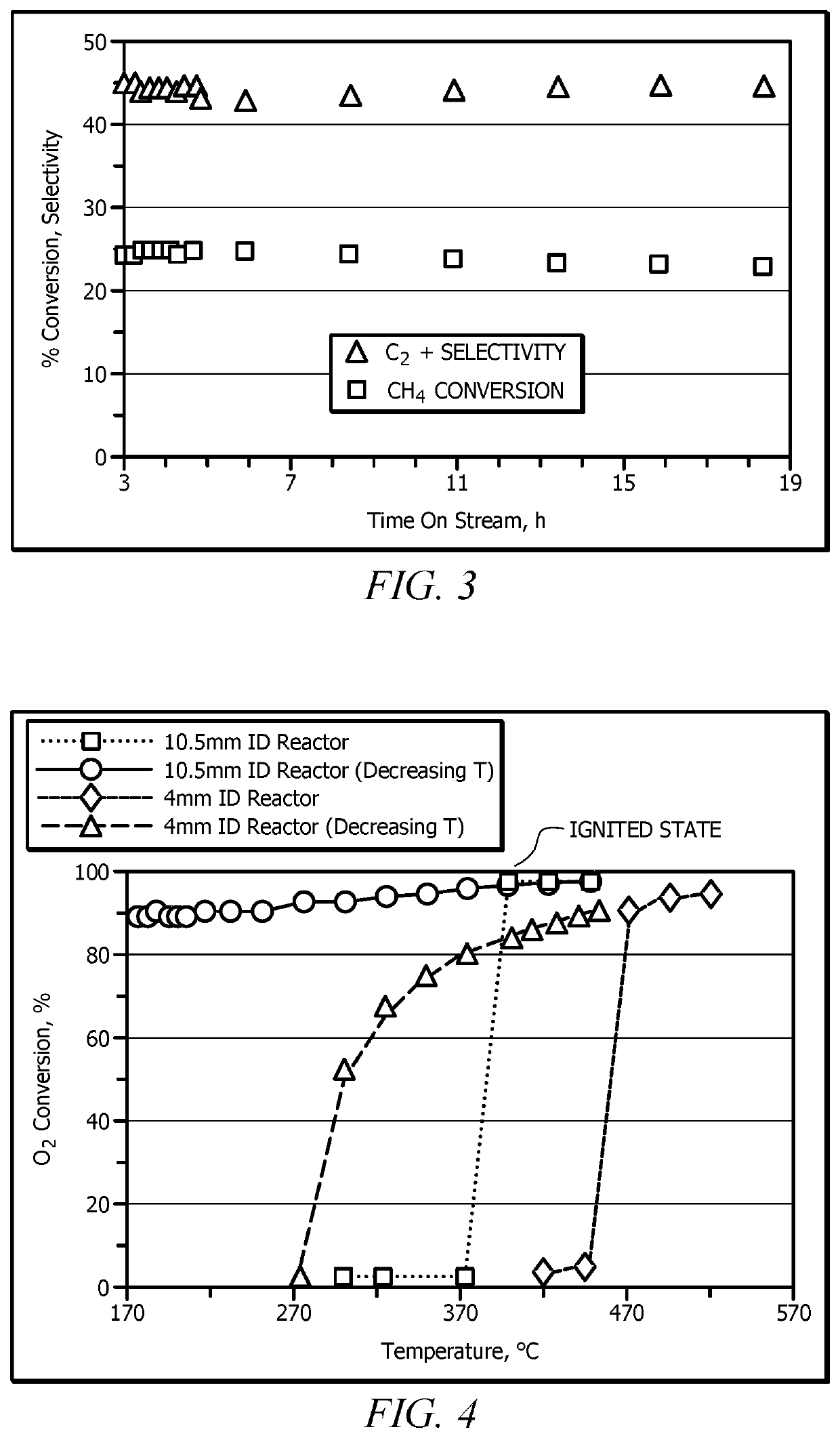
[0072]A 10.5 mm I.D. quartz reactor was used as the adiabatic reactor. A gaseous feed mixture that included reactant gases CH4 and O2 at a CH4:O2 molar ratio of 20:1 was introduced to the adiabatic reactor. The gaseous mixture was preheated to about 550-600° C. and had a residence time of from about 0.1 milliseconds to about 100 milliseconds in the catalyst bed that included a La2O3 / CeO2 catalyst having a La / Ce wt. ratio of 15. The feed composition and feed temperature during start up were changed in small steps simultaneously to a final CH4:O2 molar ratio of 4 to 5, and to an ambient feed temperature (e.g., less than 20° C.). The outlet gas from the reactor was determined by GC analysis to include C2 and higher hydrocarbons and syngas composition, such as C2H4, C2H6, CH4, CO, H2, CO2 and H2O. A steady performance for the duration of experiment (about 20 hours) was achieved. The selectivity to C2...
example 2
Comparative Example of Example 1—No Change in Startup Procedures
[0073]As a comparative example, an experiment performed with the same catalyst and reactor with the final conditions of Example 1 being used as the start-up and final conditions. Negligible methane conversion (<1%) was observed.
example 3
Comparative Example of Example 1—Change in Temperature
[0074]As a comparative example, an experiment performed with the same catalyst and reactor and at same final conditions of Example 1 was performed, except that only one parameter i.e., temperature was varied by keeping feed ratio constant at 4 and residence time of 7 milliseconds. The reaction was sustained when the feed temperature was reduced to about 160° C., but quickly died upon reducing the temperature to ambient. ‘Residence time’ refers to the contact time of the flowing gases (at reaction conditions) in the catalyst bed and is defined as the ratio of void volume in the catalyst bed to the actual volumetric flow rate under reactive conditions.
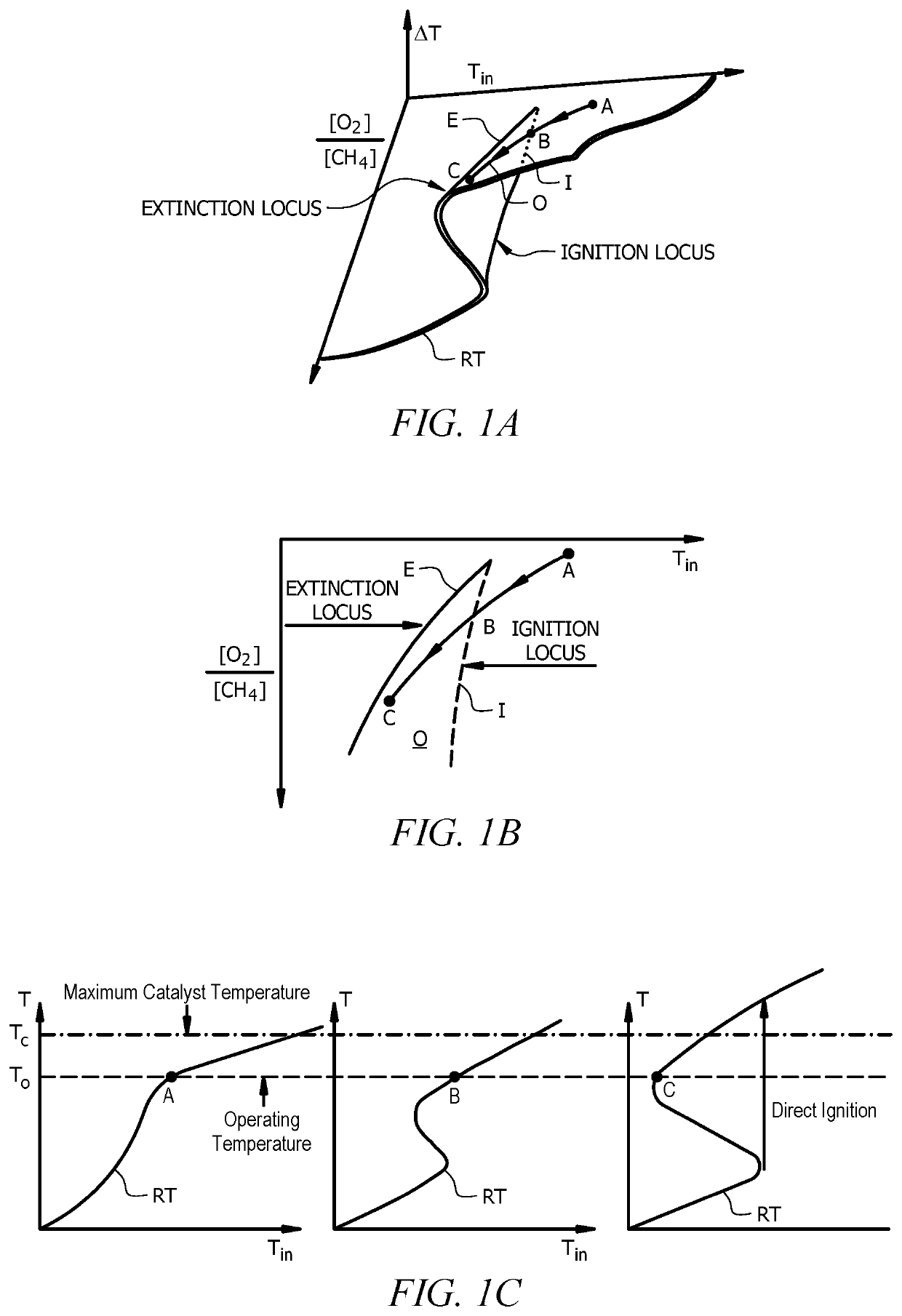
[0075]Comparison of Example 1 to Comparative Examples 2 and 3 demonstrated that varying two parameters during startup (i.e., change in molar ratio and feed temperature) provided a desired steady state condition and conversion of methane instead of little to no conversion of methane (E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com