Corrosion inhibitor
a corrosion inhibitor and corrosion inhibitor technology, applied in anti-corrosion paints, polyurea/polyurethane coatings, coatings, etc., can solve the problems of inability to meet environmental requirements, invariably less effective than chromate counterparts, and achieve improved corrosion resistance, prevent further corrosion, and prevent further corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
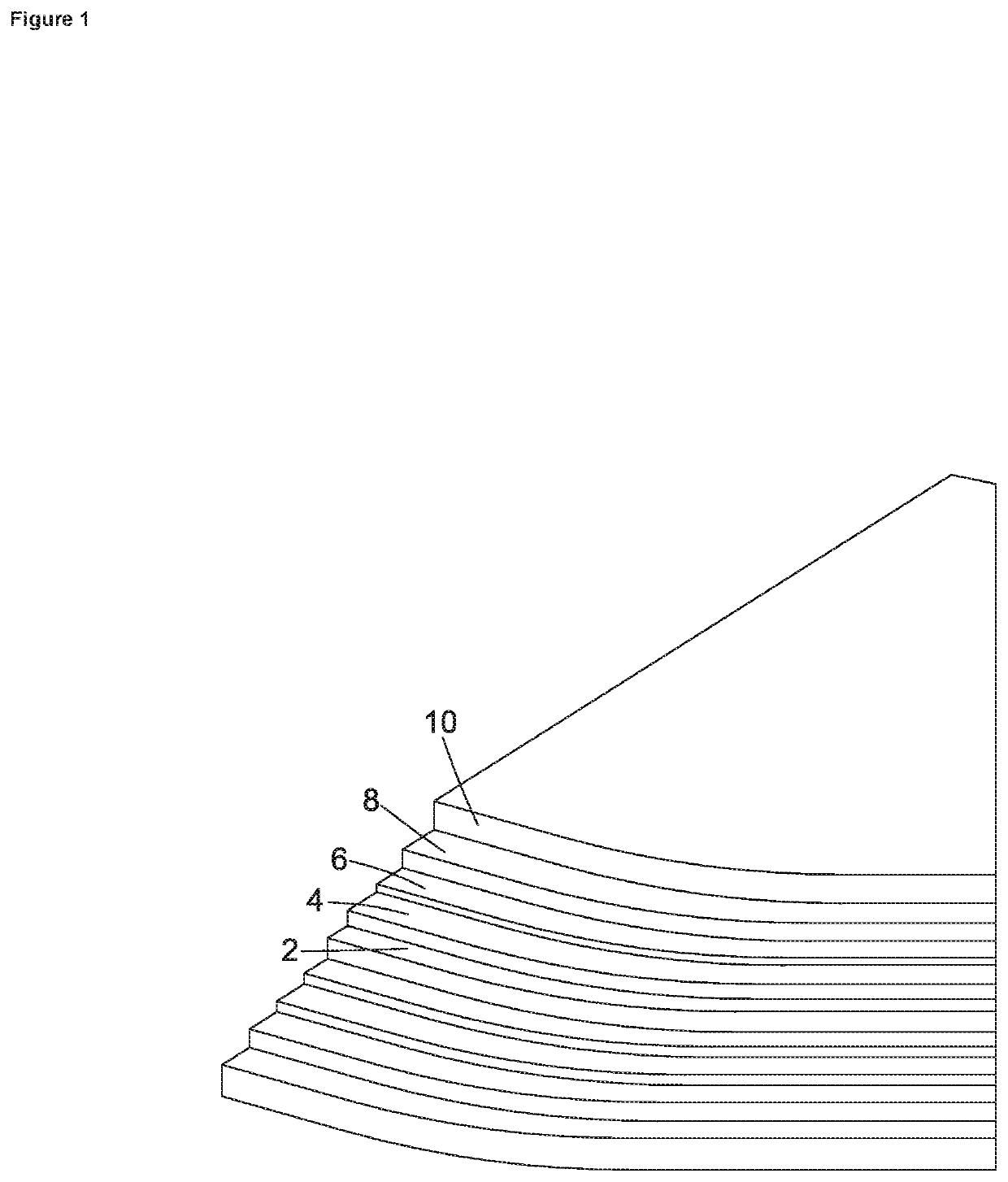
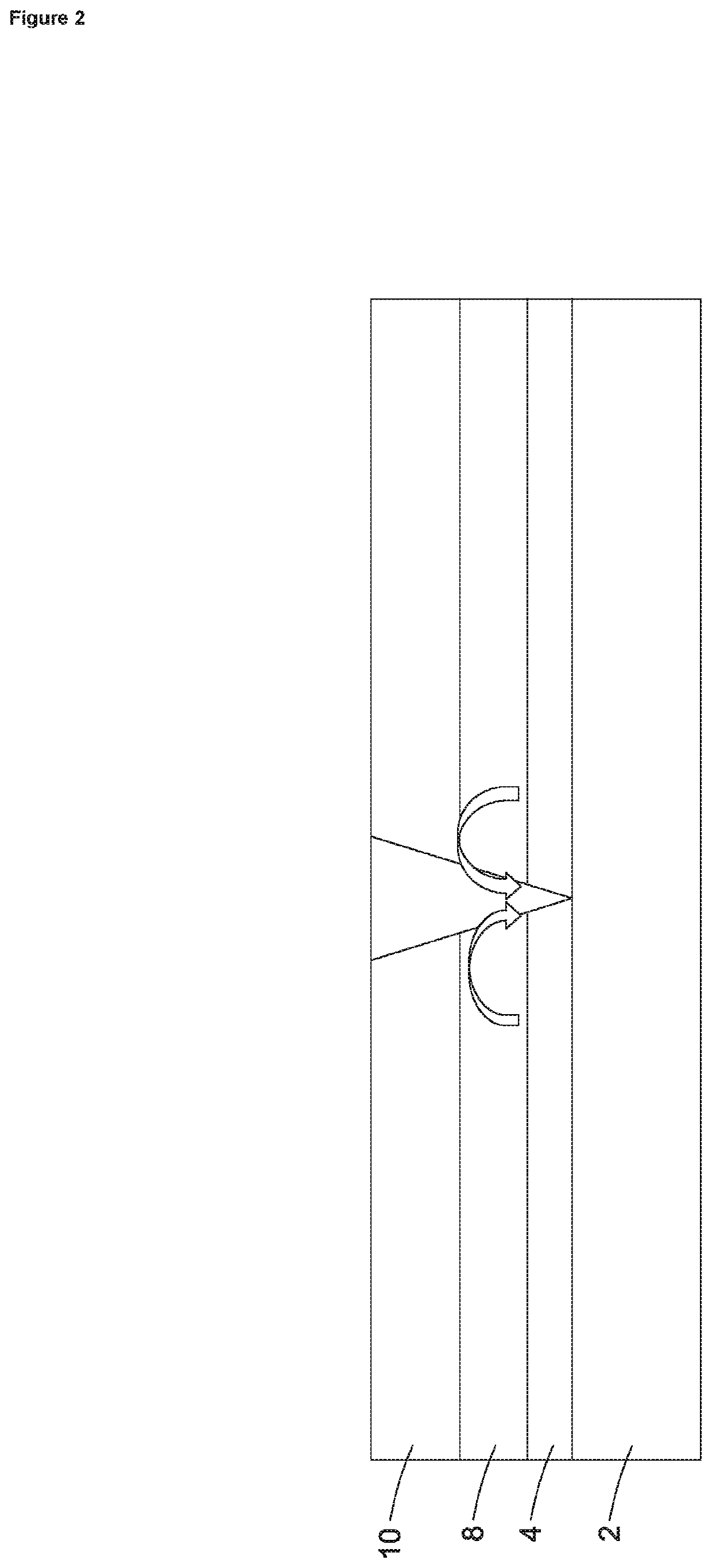
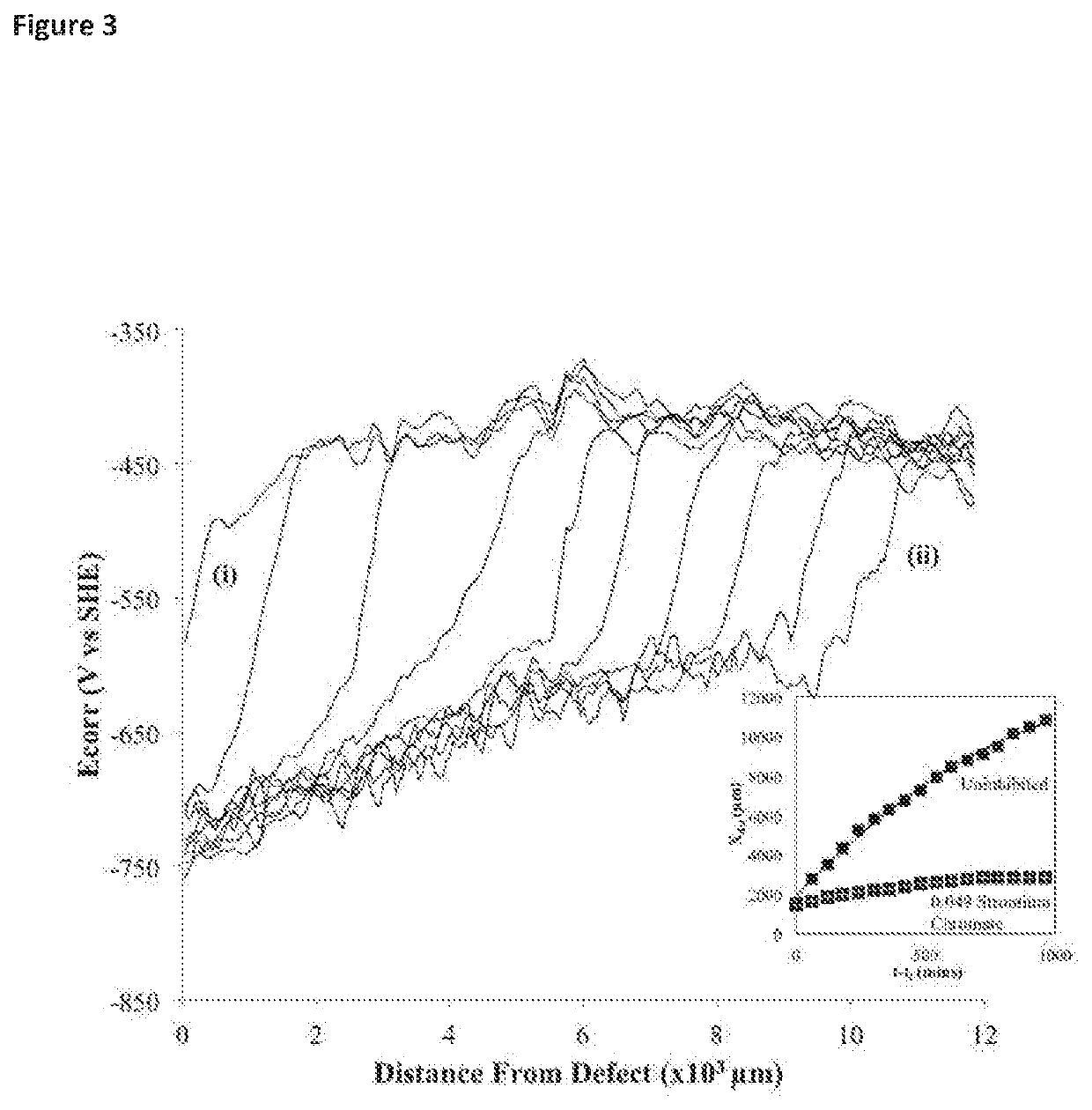
[0047]The present invention has been developed to provide a smart-release corrosion inhibitor which has particular but not exclusive application in the protection of galvanised steel from corrosion. The inhibitor, which is usually applied as a primer to a metal surface in liquid form at room temperature and pressure contains an organic ion, preferably an azole, and even more preferably benzotriazolate (BTA). This is added to an ion exchange matrix. The ion exchange resin matrix in one embodiment is a divinylbenzene copolymer with a sulphonate functional group as shown below. The benzene ring with the three nitrogen atoms is benzatriazolate and is positively charged due to extra hydrogen cation. The ion exchange resin matrix is the remainder and is shown as being negatively charged.
[0048]The corrosion inhibitor structure is formed of repeating units of the ion exchange resin with a sulphonated group having a negative charge to hold the corrosion inhibiting cation of protonated benzot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particulate size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particulate size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com