Medical apparatus and method for sterilizing medical apparatus
a medical kit and sterilisation technology, applied in the field of sterilising medical kits or utensils, can solve the problems of difficult to predict specifically what types of medical devices and/or medicaments to use, difficult situation, and not being able to fill pre-filled syringes, so as to prevent any bacterial contamination and facilitate sterilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
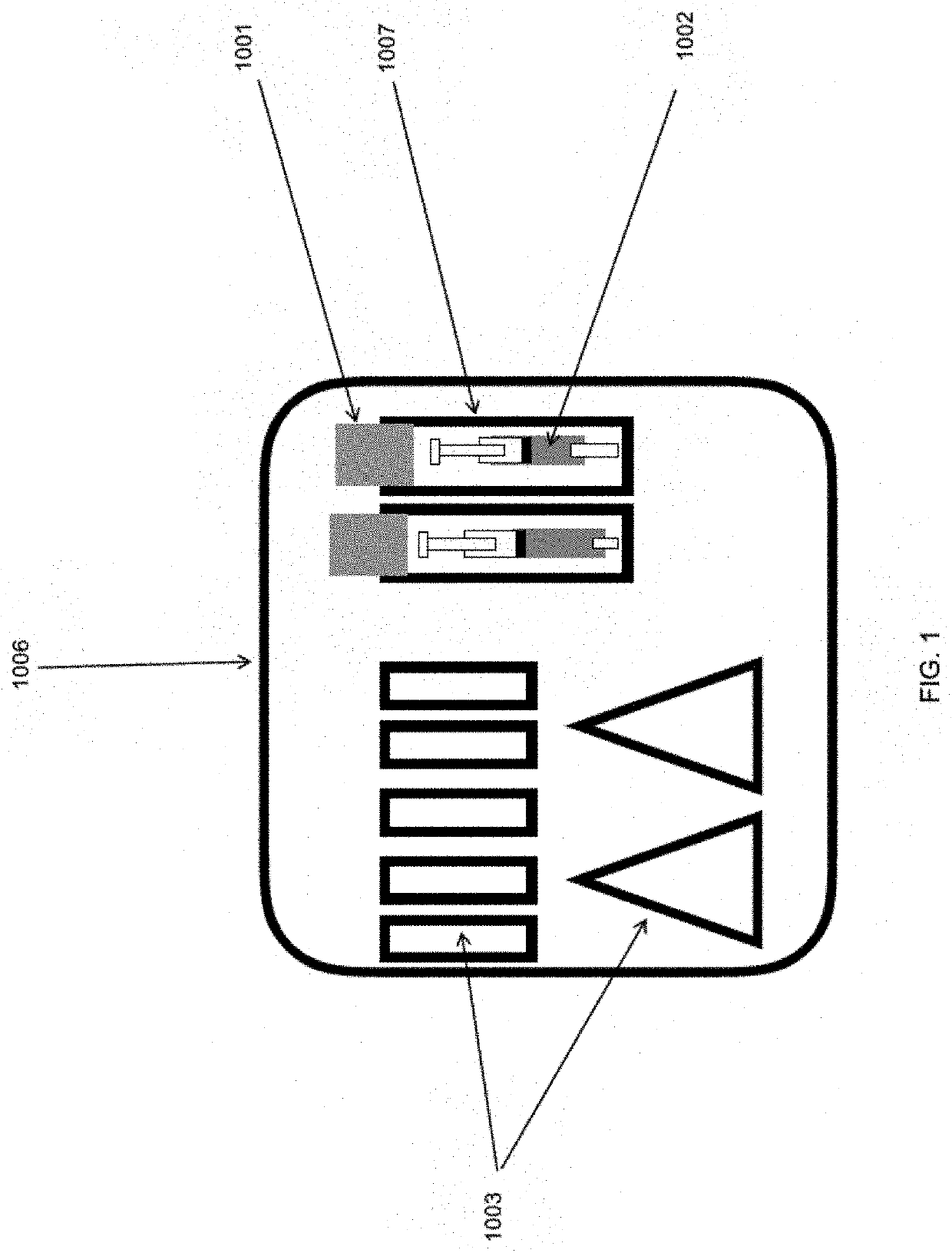
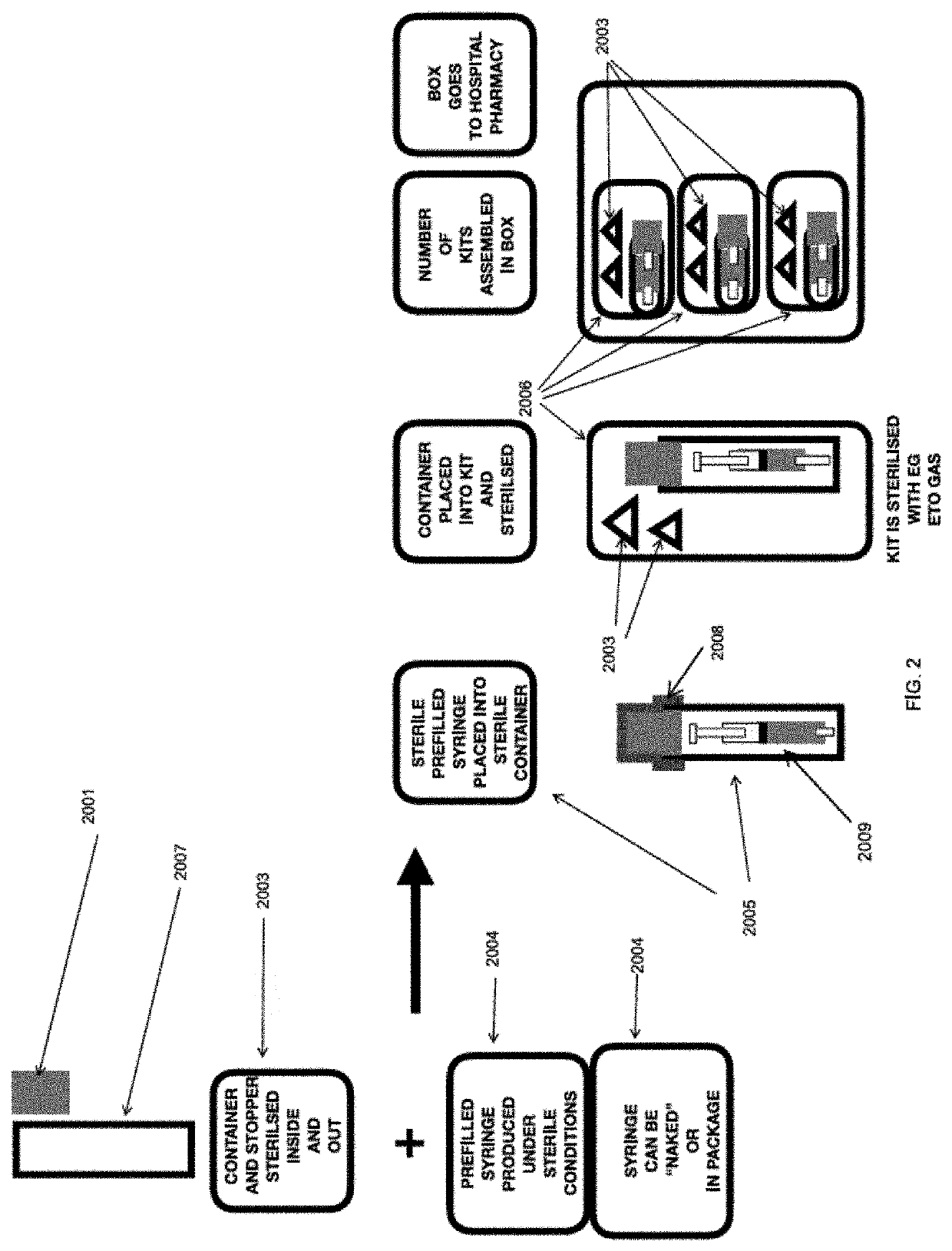
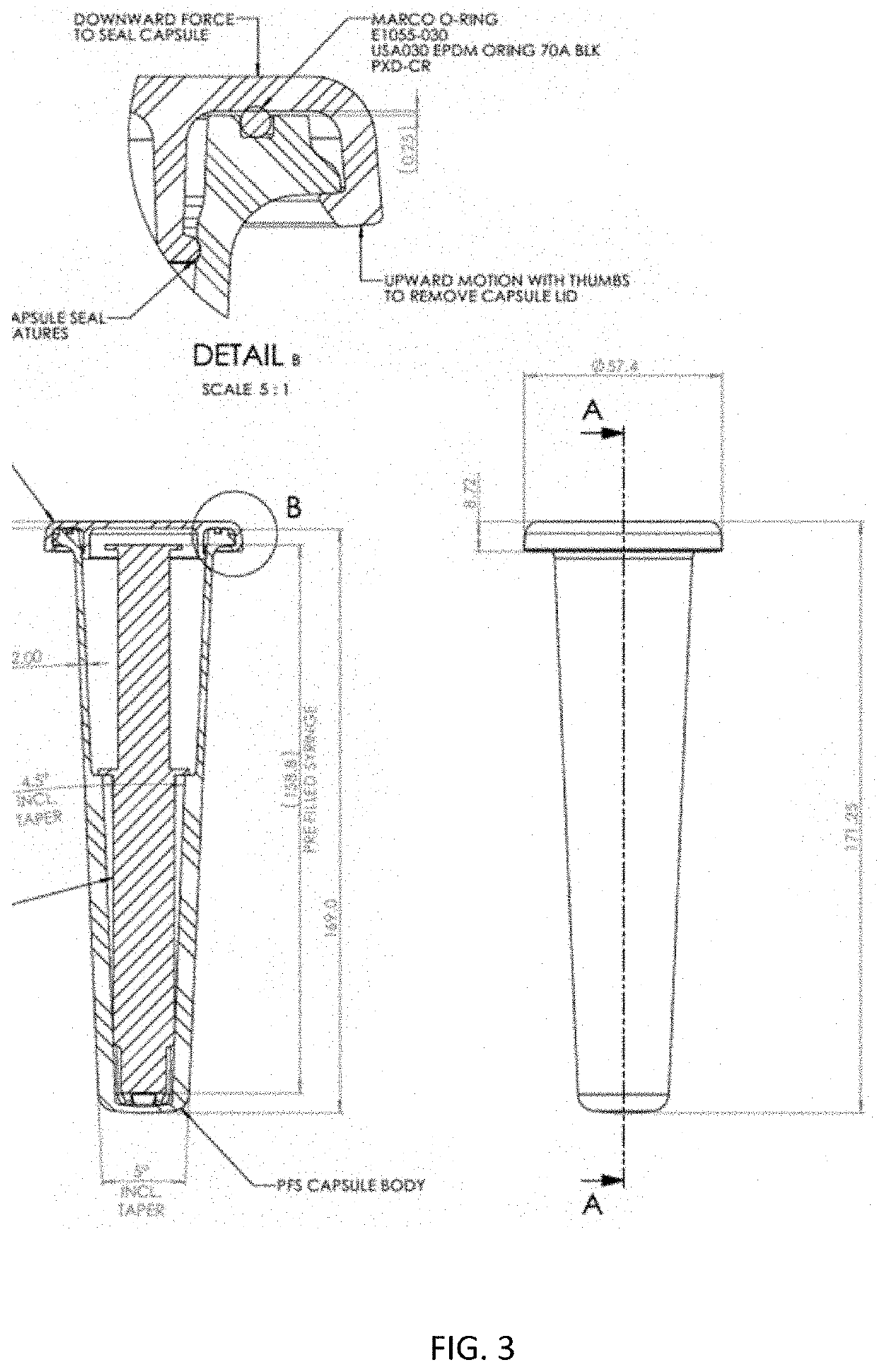
[0097]A syringe having a polypropylene body was filled with 20 ml sterile 0.9% saline solution compliant with the US Pharmacoepia. The syringe was then packaged in a protective container (glass vial) together with an ethylene oxide (ETO) detector strip (which is red at the start and turns green on exposure to ETO). The vial was then stoppered tightly with a rubber stopper and a ring pull over holder. There existed a tight fit of the stopper without defects so that the ETO sterilant will not ingress.
[0098]As a supplementary barrier, an optional aluminum foil was applied over the stopper of the vial and also over the crevice at the boundary of the cap and the vial. The foil was tightly wrapped around the protective container and was additionally provided with adhesive to ensure an intimate and durable connection with the protective container.
[0099]A kit comprising the protective container was then assembled and packaged in a surgical wrap material comprising two layers of SMS bonded t...
example 2
[0112]The following are examples of steps to prepare kits for an end user.
Embodiment AEmbodiment BEmbodiment CEmbodiment DSyringe (“syr”)Syringe exposed toSyringe exposed toSyringe exposed toexposed toETO as SecondaryETO as TertiaryETO as TertiaryPrimary(Final) Sterilization(Final) Sterilization(Final) SterilizationSterilization cycleStepStepSteponlyNS PP Syr + NS TipNS PP Syr + NS TipNS PP Syr + NS TipPrefilled flushCap + Sterile 0.9%Cap + Sterile 0.9%Cap + Sterile 0.9%syringe from marketSod Chloride USPSod Chloride USPSod Chloride USPFill Fill Fill (May be, forinto PP Syr viainto PP Syr viainto PP Syr viaexample, sterilizedpump-->push salinepump-->push saline intopump-->push salinevia irradiation,into syr; Pullsyr; Pull force-->pullinto syr; Pullsteam, autoclave orforce-->pull plungerplunger up to suckforce-->pull plungerdry / moist heat orup to suck saline in;saline inup to suck saline inother means)Overwrap syrPlace Syr in containerOverwrap syr w / dustw / dust cover or in(metal or gl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com