Improved therapeutic method for rare ocular diseases by gene replacement
a gene replacement and rare ocular disease technology, applied in the direction of genetic material ingredients, peptide sources, peptide sources, etc., can solve the problems of limited number of therapeutic approaches initiated for these dominant forms, and the dominant forms of pr degeneration are more difficult to cur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0114]Material and Methods Animals
[0115]Male and female adult (3-8 weeks old) C57BL / 6J, CrxRip / + and Tg(CRXR41W) mices, and male and female young (2 weeks old) rd10 mices were produced in the laboratory animal facilities (Animalerie central-campus CNRS Gif sur Yvette).
[0116]All animal experiments were carried out according to European guidelines for the care and use of experimental animals, and were approved by the regional ethics committee (CEEA59).
Vectors
[0117]rAAV2 / 5 vectors expressing green fluorescent protein (GFP) or human cone-rod homeobox protein (CRX) under the control of the human rhodopsin kinase (GRK1) promotor were produced.
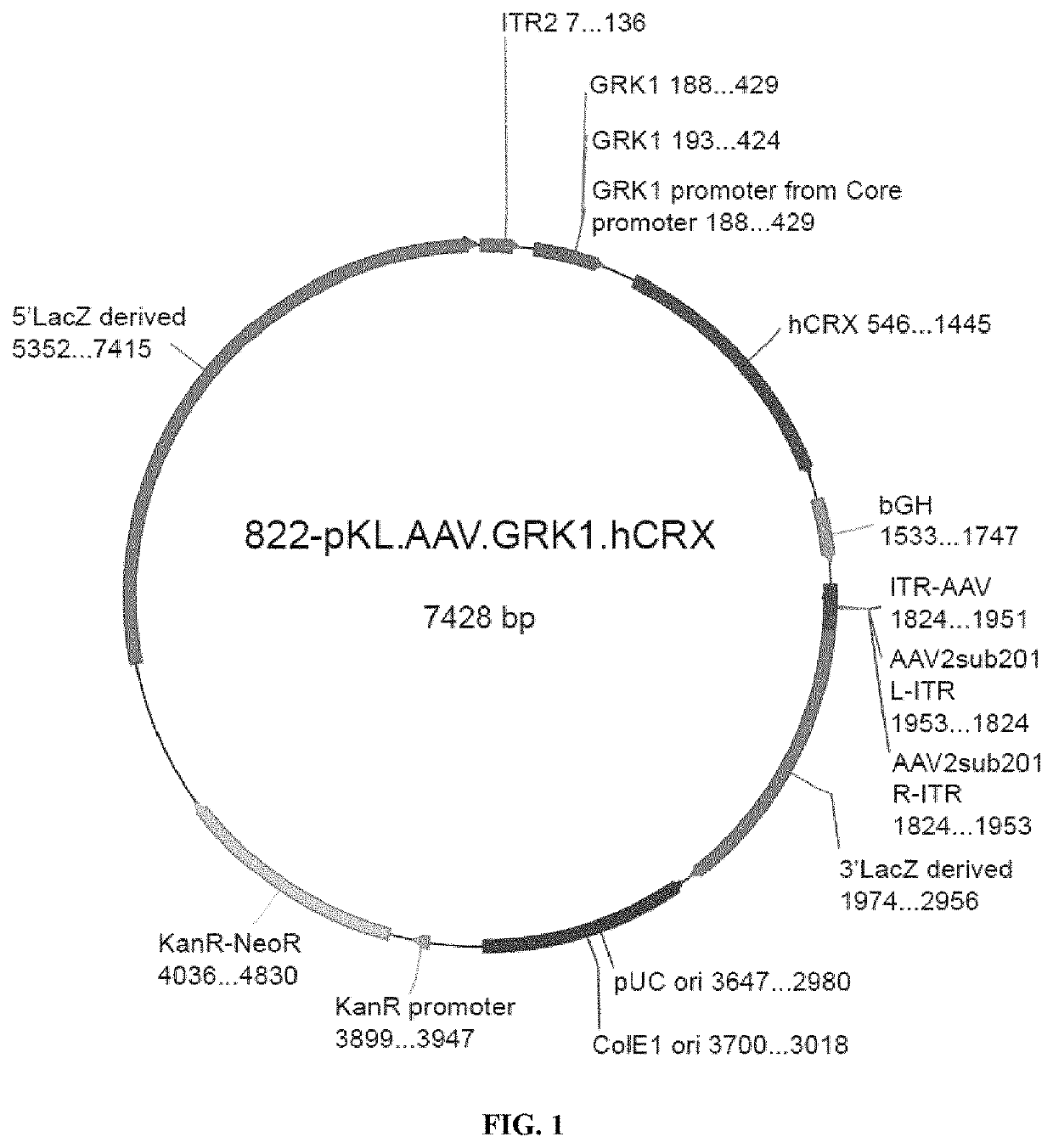
1. Vector Plasmid Cloning (822-pKL.AAV.GRK1.hCRX Vector #6556 Batch)
[0118]The vector plasmid was constructed in 2 phases as described below:[0119]First, the promoter GRK1 was extracted from the 754-pAAV.GRK1.eGFP.wpre.bGH by double digestion SpeI (position 185) and EcoR1 (position 427) and inserted in the 778-pKL.AAV.CAG.bGH vector plasmid in advance...
example 2
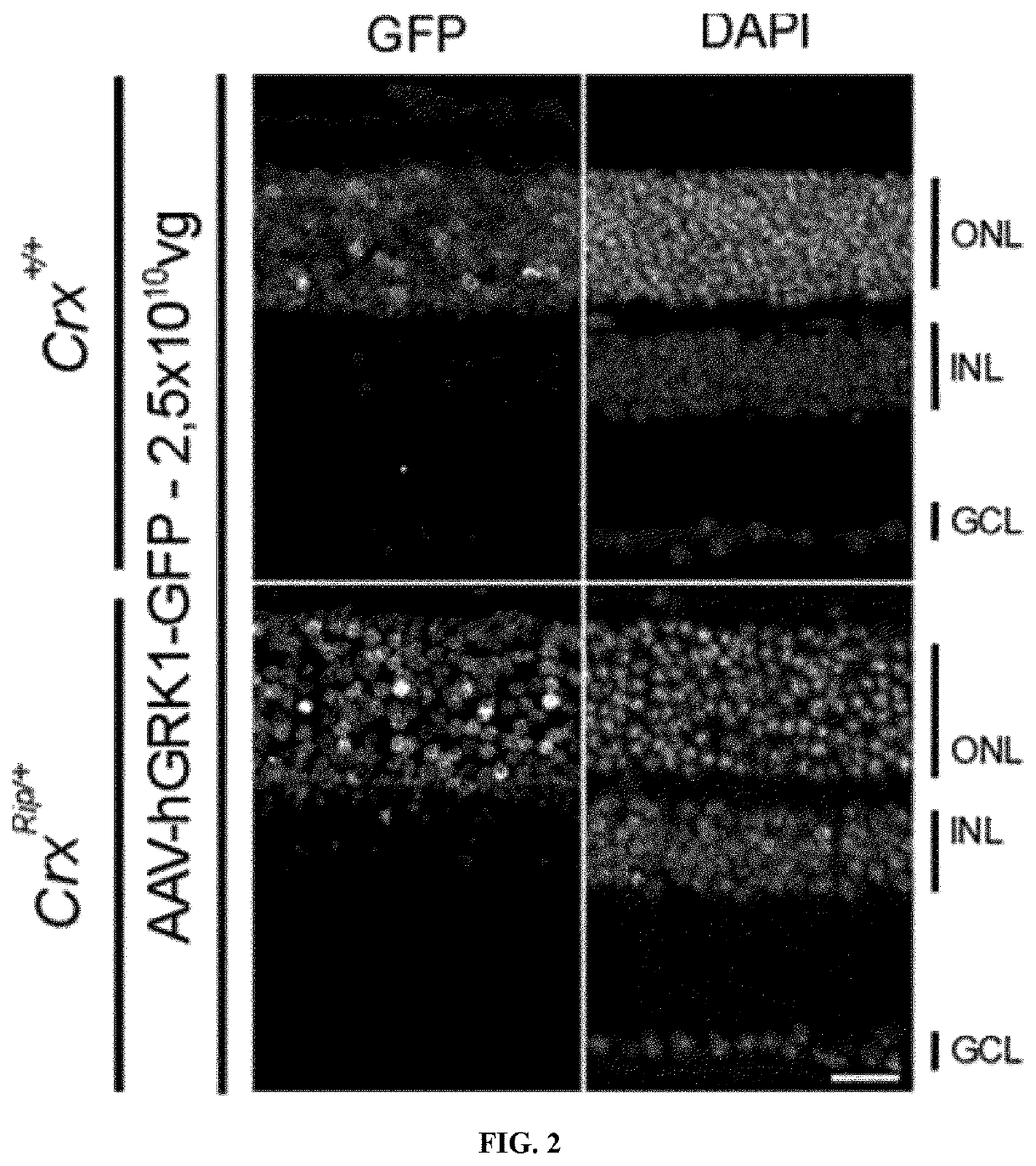
[0148]Selection of the promoter and the serotypes of the AAV vector to be used to efficiently transduced photoreceptors in CRX-associated retinopathy mouse models.
[0149]Several AAV serotypes and promoters have been tested and showed transduction of mature photoreceptors in adults. In the case of CrxRip / + retina, the outer nuclear layer contains only immature cone-like photoreceptors and therefore AAV vectors commonly used to treat differentiated photoreceptors may not be as efficient. Therefore, we tested several serotypes expressing GFP driven by a photoreceptor-specific promoter, which directs gene expression in both rod and cone photoreceptors. The human Rhodopsin kinase 1 (GRK1) promoter was selected because i) it is already well characterized to express genes in both rod and cone photoreceptors ii) it is already used in clinical trial (PDE6B) iii) It is active in our mouse models based on our published transcriptomic data. For the serotype, we selected the AAV2 / 5 vector describ...
example 3
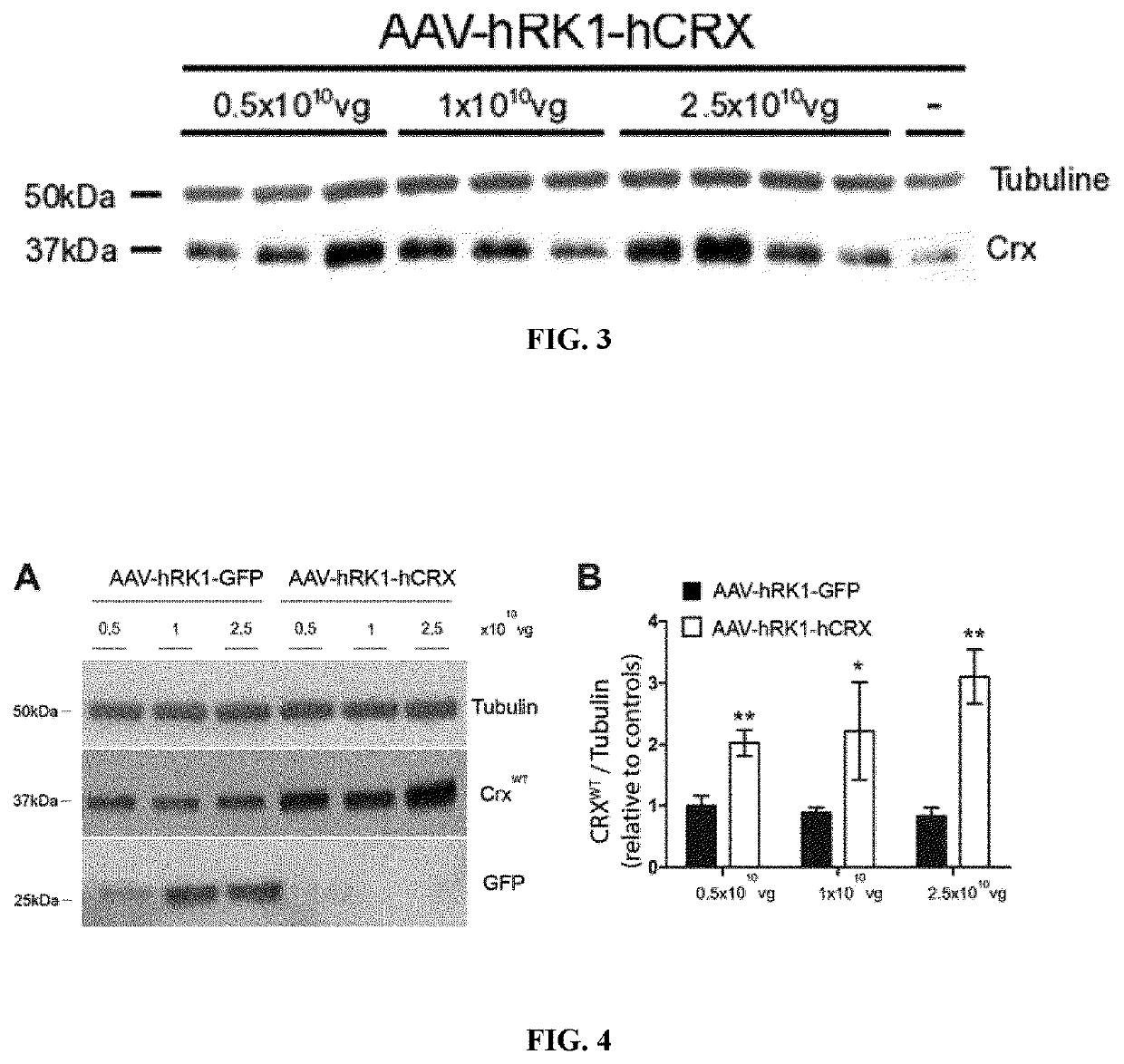
[0152]Quantification of CRX expression driven by GRK1 promoter using an AAV2 / 5 vector.
[0153]In order to verify that the product (CRX-expressing AAV vector or AAV-CRX) is able to produce CRX protein, a series of subretinal injections was performed in CrxRip / + retina with three different doses (0.5·1010, 1·1010 and 2.5·1010 vg) and CRX expression was assessed 14 days later. At all three tested doses, increased expression of CRX was observed. The amount produced was more dependent of the injection than the dose used (FIG. 3). This experiment was repeated when the subretinal injection technic was fully mastered but this time GFP-expressing vector (AAV-GFP) was injected as control (FIG. 4) at three different doses (0.5·1010, 1·1010 and 2.5·1010 vg). CRX relative expression was quantified after tubulin normalization and reported to endogenous CRX expression following AAV-GFP injection at 0.5·1010 vg. CRX expression was clearly significantly increased after AAV-CRX injection proportionally...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com